Abstract
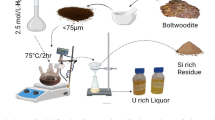
To improve the utilisation of uranium resources while reducing the radioactive contamination of uranium slag by 226Ra, the effects of various factors on uranium–radium co-leaching from uranium ore were investigated. For nitric acid agitation leaching, the optimal performance was achieved using 80 mesh ore in 4 mol L−1 nitric acid at 40 °C for 4 h with a liquid-to-solid ratio of 3. Under these conditions, the uranium leaching rate was > 99% and the radium leaching rate was > 93% with standard deviations of < 0.04% and < 0.7%, respectively. Thus, this approach realises simultaneous uranium and radium leaching at high rates.

Similar content being viewed by others
References
Zhang S et al (2021) Cancer incidence and mortality in China. J Natl Cancer Cent 1:2–11
XiaDong CX et al (2022) Cancer statistics in China and the United States, 2022: Profiles, trends, and determinants. Chin Med J 135:584–590
Królicki L et al (2020) 225Ac- and 213Bi-substance P analogues for glioma therapy. Semin Nucl Med 50:141–151
Venkatesh M, Kang KW (2021) Medicine radionuclides used in nuclear medicine. In: Greenspan E (ed) Encyclopedia of nuclear energy. Elsevier, Oxford
Seidl C (2014) Radioimmunotherapy with α-particle-emitting radionuclides. Immunotherapy 6:431–458
Ismail AF, Yim MS (2015) Investigation of activated carbon absorbent electrode for electrosorption-based uranium extraction from seawater. Nucl Eng Technol 47(5):579–587
Gabriel S et al (2013) A critical assessment of global uranium resources, including uranium in phosphate rocks, and the possible impact of uranium shortages on nuclear power fleets. Ann Nucl Energy 58(8):213–220
Mazher AK (2009) A review of uranium economics. Int J Nucl Gov Econ Ecol 2(4):337
Amaral ECS et al (1985) Pre-operational environmental survey at the uranium mine and mill site, Poços de Caldas, Minas Gerais Brazil. Sci Total Environ 42:257–266
Ballerstedt H et al (2017) Approaches for eliminating bacteria introduced during in situ bioleaching of fractured sulfidic ores in deep subsurface. Solid State Phenom 262:70–74
Carvalho IG et al (2005) Environmental impact of uranium mining and ore processing in the Lagoa Real District, Bahia Brazil. Environ Sci Technol 39:8646–8652
Ding D et al (2010) Two stage column leaching of uranium from uraninite ore. Adv Sci Lett 5:96–100
Ghazala RA (2019) Application of the produced microbial citric acid as a leachate for uranium from El-Sebaiya phosphate rock. J Radiat Res Appl Sci 12:78–86
Hefnawy MA et al (2002) Fungal leaching of uranium from its geological ores in Alloga Area, West Central Sinai Egypt. J Biol Sci 2:346–350
Ryon AD et al (1977) Nitric acid leaching of radium and other significant radionuclides from uranium ores and tailings. Oak Ridge Oak Ridge National Lab, Oak Ridge
Sethy NK et al (2014) A simple method for calibration of Lucas scintillation cell counting system for measurement of 226Ra and 222Rn. J Radiat Res Appl 7:472–477
Barker E et al (2017) Determination of 226Ra in solid samples of few milligrams after mineralization and measurement by solid scintillation. J Radioanal Nucl Chem 314:353–358
Durecova A et al (1997) Contribution to the simultaneous determination of 228Ra and 226Ra by using 2M’s EMPORETM radium rad disks. J Radioanal Nucl Chem 223:225–228
Lucas HF et al (1990) Methods for measuring radium isotopes: emanometry. In: Chapter 3–2 in “The environmental behaviour of radium”, Technical Reports Series No.310, vol.1. International atomic energy agency, Vienna, pp 149–172
Funding
This work is supported by ‘Key technology research and scale up production of high purity 226Ra from uranium ore’ and ‘Isolation of trivalent lanthanides from water-soluble bis-triazolopyridine’(Grant No. 200SJY011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai, Z., Zhao, X., Zhang, J. et al. Optimisation of uranium–radium co-leaching from uranium ore. J Radioanal Nucl Chem 332, 1841–1845 (2023). https://doi.org/10.1007/s10967-023-08892-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08892-7