Abstract
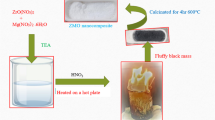
In this research, composites of ZnO in the form of flowers with ZnFe Prussian blue analogues were prepared by in situ synthesis, and found the synthesis conditions with maximum removal efficiency. The flower-like ZnO enhances the dispersion of the ZnHCF nanoparticles, which means that the Prussian blue analogue has higher adsorption utilisation. In addition, various adsorption experiments revealed that the adsorbent has the best adsorption capacity under neutral aqueous solution conditions and is capable of high removal rate adsorption in multi-ion aqueous solution systems. Apart from this, the adsorption process satisfies a pseudo-second-order kinetic model, indicating that chemisorption dominates, and the Langermuir adsorption model suggests that adsorption occurs on a monolayer and is favourable, with a maximum adsorption capacity of 322.4 mg/g, while adsorption is a spontaneous, heat-absorbing process. Finally, a comparison with other Prussian blue analogues revealed the potential of flower-like ZnO/ZnHCF for efficient removal of Cs+ from water.









Similar content being viewed by others
References
Miyazaki M, Taigawa K, Murakami M (2016) After Fukushima: creating a dialogue. Science 352:666–666. https://doi.org/10.1126/science.352.6286.666-b
Querfeld R, Pasi AE, Shozugawa K, Vockenhuber C, Synal HA, Steier P, Steinhauser G (2019) Radionuclides in surface waters around the damaged Fukushima Daiichi NPP one month after the accident: evidence of significant tritium release into the environment. J Sci Total Environ 689:451–456. https://doi.org/10.1016/j.scitotenv.2019.06.362
Goudarzi M, Weber W, Mak TD, Wang MX, Chung JJ, Doyle-Eisele M, Melo D, Brenner DJ, Guilmette RA, Fornace AJ (2014) Development of urinary biomarkers for internal exposure by Cesium-137 using a metabolomics approach in mice. Radiat Res 181(1):54–64. https://doi.org/10.1667/rr13479.1
Lei ZW, Li XW, Huang PW, Hu HM, Li Z, Zhang QW (2019) Mechanochemical activation of antigorite to provide active magnesium for precipitating cesium from the existences of potassium and sodium. J Appl Clay Sci 168:223–229. https://doi.org/10.1016/j.clay.2018.11.015
Zhang JF, Yang LR, Dong TT, Pan F, Xing HF, Liu HZ (2018) Kinetics-controlled separation intensification for cesium and rubidium isolation from salt lake Brine. Ind Eng Chem Res 57:4399–4406. https://doi.org/10.1021/acs.iecr.7b04820
Su JY, Jin GP, Chen T, Liu XD, Chen CN, Tian JJ (2017) The characterization and application of prussian blue at graphene coated carbon fibers in a separated adsorption and electrically switched ion exchange desorption processes of cesium. J Electrochim Acta 230:399–406. https://doi.org/10.1016/j.electacta.2017.02.027
Hao S, Geng YZ, Jia ZQ (2018) UV pre-activation/thermal initiated grafting of caffeic acid on PVDF for preparation of adsorptive membranes for cesium. J React Funct Polym 132:120–126. https://doi.org/10.1016/j.reactfunctpolym.2018.09.020
Tan ZY, Dong L, Huang ZY, Du L, Wang XL (2016) A theoretical study on the selective adsorption of NH4+ and Cs+ on the phosphomolybdate ion. J. Colloids Surf, A 502:74–80. https://doi.org/10.1016/j.colsurfa.2016.05.019
Torad NL, Naito M, Tatami J, Endo A, Leo SY, Ishihara S, Wu KCW, Wakihara T, Yamauchi Y (2014) Highly crystallized nanometer-sized zeolite a with large Cs adsorption capability for the decontamination of water. J. Chem-Asian 9:759–763. https://doi.org/10.1002/asia.201301132
Xiang Y, Hou L, Liu JM, Li J, Lu ZY, Niu YH (2021) Adsorption and enrichment of simulated Cs-137 in geopolymer foams. J Environ Chem Eng 9(4):105733. https://doi.org/10.1016/j.jece.2021.105733
Xiang SL, Zhang X, Tao QQ, Dai Y (2019) Adsorption of cesium on mesoporous SBA-15 material containing embedded copper hexacyanoferrate. J Radioanal Nucl Chem 320(3):609–619. https://doi.org/10.1007/s10967-019-06523-8
Parajuli D, Kitajima A, Takahashi A, Tanaka H, Ogawa H, Hakuta Y, Yoshino K, Funahashi T, Yamaguchi M, Osada M, Kawamoto T (2016) Application of Prussian blue nanoparticles for the radioactive Cs decontamination in Fukushima region. J Environ Radioact 151:233–237. https://doi.org/10.1016/j.jenvrad.2015.10.014
Chen SQ, Hu JY, Han SJ, Guo YF, Belzile N, Deng TL (2020) A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade. Sep Purif Technol 251:117340. https://doi.org/10.1016/j.seppur.2020.117340
Yang HJ, Li HY, Zhai JL, Sun L, Zhao Y, Yu HW (2014) Magnetic prussian blue/graphene oxide nanocomposites caged in calcium alginate microbeads for elimination of cesium ions from water and soil. Chem Eng J 246:10–19. https://doi.org/10.1016/j.cej.2014.02.060
Chen SQ, Yang XN, Wang Z, Hu JY, Han SJ, Guo YF, Deng TL (2021) Prussian blue analogs-based layered double hydroxides for highly efficient Cs plus removal from wastewater. J Hazard Mater 410:124608. https://doi.org/10.1016/j.jhazmat.2020.124608
Wang PH, Chang YR, Chen ML, Lo YK, Lee DJ (2021) Shape stable poly(vinyl alcohol) hydrogels with immobilized metal hexacyanoferrates for cesium removal from waters. Environ Sci Pollut Res 29(9):12427–12433. https://doi.org/10.1007/s11356-021-14937-9
Saito N, Haneda H (2012) Hierarchical structures of ZnO spherical particles synthesized solvothermally. Sci Technol Adv Mater 12(6):064707. https://doi.org/10.1088/1468-6996/12/6/064707
Rajesh UC, Wang JF, Prescott S, Tsuzuki T, Rawatt DS (2015) RGO/ZnO Nanocomposite: an efficient, sustainable, heterogeneous, amphiphilic catalyst for synthesis of 3-substituted indoles in water. ACS Sustain Chem Eng. https://doi.org/10.1021/sc500594w
Silva MNT, Ardisson JD, Fabris JD, Nossol E (2020) Zinc Hexacyanoferrate/multi-walled carbon nanotubes films for rechargeable aqueous batteries. J Braz Chem Soc 31(9):1787–1795. https://doi.org/10.21577/0103-5053.20200064
Lee HK, Choi JW, Kim JH, Kim CR, Choi SJ (2021) Simultaneous selective removal of cesium and cobalt from waterusing calcium alginate-zinc ferrocyanide-Cyanex 272 composite beads. Environ Sci Pollut Res 28(31):42014–42023. https://doi.org/10.1007/s11356-021-13342-6
Atchudan R, Edison TNJI, Mani S, Perumal S, Vinodh R, Thirunavukkarasu S, Lee YR (2020) Facile synthesis of novel nitrogen-doped carbon dots adorned zinc oxide composite for photodegradation of methylene blue. Dalton Trans 49(48):17725–17736. https://doi.org/10.1039/d0dt02756a
Arjun K, Karthikeyan B (2022) Flexible ultraviolet photodetector based on flower-like ZnO/PEDOT:PSS nanocomposites. Appl Phys A: Mater Sci Process 128(5):449. https://doi.org/10.1007/s00339-022-05516-x
Zhang LY, Chen L, Zhou XF, Liu ZP (2015) Morphology-dependent electrochemical performance of zinc hexacyanoferrate cathode for zinc-ion battery. Sci Rep 5:18263. https://doi.org/10.1038/srep18263
Olatunji M, Khandaker M, Mahmud E, Amin Y, Ademola J, Olorode D (2018) Remediation of 137Cs radionuclide in nuclear waste effluents by polymer composite: adsorption kinetics, isotherms and gamma irradiation studies. J Radioanal Nucl Chem 316(3):933–945. https://doi.org/10.1007/s10967-018-5875-4
Kim Y, Kim I, Lee TS, Lee E, Lee KJ (2018) Porous hydrogel containing Prussian blue nanoparticles for effective cesium ion adsorption in aqueous media. J Ind Eng Chem 60:465–474. https://doi.org/10.1016/j.jiec.2017.11.034
Yang HJ, Sun L, Zhai JL, Li HY, Zhao Y, Yu HW (2014) In situ controllable synthesis of magnetic Prussian blue/graphene oxide nanocomposites for removal of radioactive cesium in water. J Mater Chem 2(2):326–332. https://doi.org/10.1039/c3ta13548a
Ma C, Jiang ZZ, Han SJ, Guo YF, Deng TL (2021) Novel one-pot solvothermal synthesis of high-performance copper hexacyanoferrate for Cs+ removal from wastewater. J Chem 2021:3762917. https://doi.org/10.1155/2021/3762917
Tan IAW, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154(1–3):337–346. https://doi.org/10.1016/j.jhazmat.2007.10.031
Simonin JP (2016) On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem Eng J 300:254–263. https://doi.org/10.1016/j.cej.2016.04.079
Lee JH, Kwak SY (2022) Mechanochemically synthesized prussian blue for efficient removal of cesium ions from aqueous solutions. ACS Omega 7(4):3222–3229. https://doi.org/10.1021/acsomega.1c05062
Mahmoud MA (2014) Kinetics and thermodynamics of aluminum oxide nanopowder as adsorbent for Fe (III) from aqueous solution. Beni-Suef Univ J Basic Appl Sci 4(2):142–149. https://doi.org/10.1016/j.bjbas.2015.05.008
Nordstrand J, Toledo-Carrillo E, Kloo L, Dutta J (2022) Sodium to cesium ions: a general ladder mechanism of ion diffusion in prussian blue analogs. Phys Chem Chem Phys 24(20):12374–12382. https://doi.org/10.1039/d2cp01156e
Tang XY, Wang SY, Zhang ZH, Li ZJ, Wang L, Yuan LY, Wang BR, Sun J, Zheng LR, Wang HQ, Shi WQ (2022) Graphene oxide/chitosan/potassium copper hexacyanoferrate(II) composite aerogel for efficient removal of cesium. Chem Eng J 444:136397. https://doi.org/10.1016/j.cej.2022.136397
Kim H, Eom HH, Kim Y, Harbottle D, Lee JW (2022) Reversible electro-mediated cesium ion removal using a zeolitic imidazolate framework derived zinc hexacyanoferrate composite. Chem Eng J 450(2):138029. https://doi.org/10.1016/j.cej.2022.138029
Feng SS, Cao X, Zheng W, Yue XL, Li XD, Li SZ, Wang XY, Feng S (2022) In-situ formed Prussian blue nanoparticles supported by porous biochar as highly efficient removal of cesium ions. J Environ Chem Eng 10(3):107972. https://doi.org/10.1016/j.jece.2022.107972
Nayl AA, Ahmed IM, Abd-Elhamid AI, Aly HF, Attallah MF (2020) Selective sorption of Cs-134 and Co-60 radioisotopes using synthetic nanocopper ferrocyanide-SiO2 materials. Sep Purif Technol 234:116060. https://doi.org/10.1016/j.seppur.2019.116060
Li JX, Zan YX, Zhang ZP, Dou ML, Wang F (2020) Prussian blue nanocubes decorated on nitrogen-doped hierarchically porous carbon network for efficient sorption of radioactive cesium. J Hazard Mater 385:121568
Naeimi S, Faghihian H (2017) Performance of novel adsorbent prepared by magnetic metal-organic framework (MOF) modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution. Sep Purif Technol 175:255–265. https://doi.org/10.1016/j.seppur.2016.11.028
Jang J, Lee DS (2016) Magnetic prussian blue nanocomposites for effective cesium removal from aqueous solution. Ind Eng Chem Res 55(13):3852–3860. https://doi.org/10.1021/acs.iecr.6b00112
Xu L, Tao QQ, Dai Y (2022) Separation of cesium using magnetic copper Hexacyanoferrate/Biochar/Fe3O4. Clean-Soil Air Water 50(9):2100347. https://doi.org/10.1002/clen.20210034
Torad NL, Hu M, Imura M, Naito M, Yamauchi Y (2012) Large Cs adsorption capability of nanostructured Prussian Blue particles with high accessible surface areas. J Mater Chem 22(35):18261–18267. https://doi.org/10.1039/c2jm32805d
Acknowledgements
This research is financially supported by The key scientific research projects of the Southwest University of Science and Technology (No.17zx910201), Longshan academic talent research supporting program of SWUST (No. 18LZX437 and No. 18LZXT09) and Sichuan science and technology program (No. 2017TD0020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, F., Zhang, Z. & Xu, G. In situ synthesis of flower-like ZnO/ZnHCF composites for cesium removal from water. J Radioanal Nucl Chem 332, 2123–2134 (2023). https://doi.org/10.1007/s10967-023-08873-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08873-w