Abstract
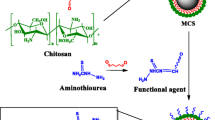
To effectively remove cobalt, magnetic biochar was modified with chitosan and ethylenediaminetetraacetic acid (CMBC–EDTA), and its properties were examined through surface area and pore size analysis, scanning electron microscopy, Fourier-transform infrared spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction analysis, and vibration sample magnetometry. Moreover, according to the Langmuir isotherm model, the maximum capacity of CMBC–EDTA reached 0.7311 mol·kg−1. Over 50% of 50 ppm Co2+ was removed within 20 min, and the adsorption equilibrium was reached after 2 h. At pH 3–7, the 20 ppm Co2+ removal efficiency was more than 90%, and it decreased rapidly below pH 2.
















Similar content being viewed by others
References
Hawkins M (2001) Why we need cobalt. Appl Earth Sci 110:66–70. https://doi.org/10.1179/aes.2001.110.2.66
Schirrmacher UO (1967) Case of cobalt poisoning. Br Med J 1:544–545
Kamiya K, Ozasa K, Akiba S, Niwa O, Kodama K, Takamura N, Zaharieva EK, Kimura Y, Wakeford R (2015) Long-term effects of radiation exposure on health. Lancet 386:469–478. https://doi.org/10.1016/S0140-6736(15)61167-9
Mendes FD, Martins AH (2004) Selective sorption of nickel and cobalt from sulphate solutions using chelating resins. Int J Miner Process 74:359–371. https://doi.org/10.1016/j.minpro.2004.04.003
Al-Shahrani H, Alakhras F, Al-Abbad E, Al-Mazaideh G, Hosseini-Bandegharaei A, Ouerfelli N (2018) Sorption of cobalt (II) ions from aqueous solutions using chemically modified chitosan. Global NEST J 20:620–627. https://doi.org/10.30955/gnj.002804
Jia F, Yin Y, Wang J (2018) Removal of cobalt ions from simulated radioactive wastewater by vacuum membrane distillation. Prog Nucl Energy 103:20–27. https://doi.org/10.1016/j.pnucene.2017.11.008
Kim H-J, Baek K, Kim B-K, Yang J-W (2005) Humic substance-enhanced ultrafiltration for removal of cobalt. J Hazard Mater 122:31–36. https://doi.org/10.1016/j.jhazmat.2005.03.043
Assaad E, Azzouz A, Nistor D, Ursu AV, Sajin T, Miron DN, Monette F, Niquette P, Hausler R (2007) Metal removal through synergic coagulation–flocculation using an optimized chitosan–montmorillonite system. Appl Clay Sci 37:258–274. https://doi.org/10.1016/j.clay.2007.02.007
Anbari RHA, Albaidani J, Alfatlawi SM, Al-Hamdani TA (2008) Removal of heavy metals from industrial water using electro-coagulation technique
Quiton KGN, Huang Y-H, Lu M-C (2022) Recovery of cobalt and copper from single- and co-contaminated simulated electroplating wastewater via carbonate and hydroxide precipitation. Sustain Environ Res 32:31. https://doi.org/10.1186/s42834-022-00140-z
Becker FL, Rodríguez D, Schwab M (2012) Magnetic removal of cobalt from waste water by ferrite co-precipitation. Procedia Mater Sci 1:644–650. https://doi.org/10.1016/j.mspro.2012.06.087
Kim JS, Keane MA (2002) The removal of iron and cobalt from aqueous solutions by ion exchange with Na-Y zeolite: batch, semi-batch and continuous operation. J Chem Technol Biotechnol 77:633–640. https://doi.org/10.1002/jctb.618
Abd El-Latif MM, Elkady MF (2011) Kinetics study and thermodynamic behavior for removing cesium, cobalt and nickel ions from aqueous solution using nano-zirconium vanadate ion exchanger. Desalination 271:41–54. https://doi.org/10.1016/j.desal.2010.12.004
Saad DR, Alismaeel ZT, Abbar AH (2020) Cobalt removal from simulated wastewaters using a novel flow-by fixed bed bio-electrochemical reactor. Chem Eng Process Process Intensif 156:108097. https://doi.org/10.1016/j.cep.2020.108097
Adiloglu S, Duban S. (2022) Removal of Cobalt, Nickel, Cadmium, and Lead from Wastewater by Phytoremediation. In: Kumar V, Thakur IS (eds) Omics Insights in Environmental Bioremediation. Springer Nature, Singapore, pp 273–300
Islam MdA, Morton DW, Johnson BB, Pramanik BK, Mainali B, Angove MJ (2018) Opportunities and constraints of using the innovative adsorbents for the removal of cobalt(II) from wastewater: a review. Environ Nanotechnol Monitor Manage 10:435–456. https://doi.org/10.1016/j.enmm.2018.10.003
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions – a review. Biores Technol 99:6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Liu Y, Lonappan L, Brar SK, Yang S (2018) Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: a review. Sci Total Environ 645:60–70. https://doi.org/10.1016/j.scitotenv.2018.07.099
Wei D, Li B, Huang H, Luo L, Zhang J, Yang Y, Guo J, Tang L, Zeng G, Zhou Y (2018) Biochar-based functional materials in the purification of agricultural wastewater: fabrication, application and future research needs. Chemosphere 197:165–180. https://doi.org/10.1016/j.chemosphere.2017.12.193
Qiu B, Tao X, Wang H, Li W, Ding X, Chu H (2021) Biochar as a low-cost adsorbent for aqueous heavy metal removal: a review. J Anal Appl Pyrolysis 155:105081. https://doi.org/10.1016/j.jaap.2021.105081
Kasera N, Kolar P, Hall SG (2022) Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: a review. Biochar 4:17. https://doi.org/10.1007/s42773-022-00145-2
Jeyasubramanian K, Thangagiri B, Sakthivel A, Dhaveethu Raja J, Seenivasan S, Vallinayagam P, Madhavan D, Malathi Devi S, Rathika B (2021) A complete review on biochar: production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 292:120243. https://doi.org/10.1016/j.fuel.2021.120243
Das S, Avasthe R, Singh R, Babu S (2014) Biochar as carbon negative in carbon credit under changing climate. Curr Sci 107:1090–1091
Yan L, Kong L, Qu Z, Li L, Shen G (2015) Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain Chem Eng 3:125–132. https://doi.org/10.1021/sc500619r
Yin Z, Xu S, Liu S, Xu S, Li J, Zhang Y (2020) A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour Technol 300:122680. https://doi.org/10.1016/j.biortech.2019.122680
Huang D, Zhang Q, Zhang C, Wang R, Deng R, Luo H, Li T, Li J, Chen S, Liu C (2020) Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline. Chem Eng J 391:123532. https://doi.org/10.1016/j.cej.2019.123532
Gómez-Pastora J, Bringas E, Ortiz I (2014) Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem Eng J 256:187–204. https://doi.org/10.1016/j.cej.2014.06.119
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27. https://doi.org/10.1016/S1381-5148(00)00038-9
Mjad ZM (2005) Advances in chitin and chitosan modification through graft copolymerization. 14:235–265
Wei YC, Hudson SM, Mayer JM, Kaplan DL (1992) The crosslinking of chitosan fibers. J Polym Sci A Polym Chem 30:2187–2193. https://doi.org/10.1002/pola.1992.080301013
Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R (2004) Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm 57:19–34. https://doi.org/10.1016/S0939-6411(03)00161-9
Saheed IO, Oh WD, Suah FBM (2021) Chitosan modifications for adsorption of pollutants—a review. J Hazardous Mater 408:124889. https://doi.org/10.1016/j.jhazmat.2020.124889
Zhang M, Liu Y, Li T, Xu W, Zheng B, Tan X, Wang H, Guo Y, Guo F, Wang S (2015) Chitosan modification of magnetic biochar produced from Eichhornia crassipes for enhanced sorption of Cr( vi ) from aqueous solution. RSC Adv 5:46955–46964. https://doi.org/10.1039/C5RA02388B
Ezzeddine Z, Batonneau-Gener I, Pouilloux Y, Hamad H, Saad Z, Kazpard V (2015) Divalent heavy metals adsorption onto different types of EDTA-modified mesoporous materials: Effectiveness and complexation rate. Microporous Mesoporous Mater 212:125–136. https://doi.org/10.1016/j.micromeso.2015.03.013
Abbas M, Kaddour S, Trari M (2014) Kinetic and equilibrium studies of cobalt adsorption on apricot stone activated carbon. J Ind Eng Chem 20:745–751. https://doi.org/10.1016/j.jiec.2013.06.030
Mohammadi SZ, Darijani Z, Karimi MA (2020) Fast and efficient removal of phenol by magnetic activated carbon-cobalt nanoparticles. J Alloys Compounds 832:154942. https://doi.org/10.1016/j.jallcom.2020.154942
Fang F, Kong L, Huang J, Wu S, Zhang K, Wang X, Sun B, Jin Z, Wang J, Huang X-J, Liu J (2014) Removal of cobalt ions from aqueous solution by an amination graphene oxide nanocomposite. J Hazard Mater 270:1–10. https://doi.org/10.1016/j.jhazmat.2014.01.031
Song W, Hu J, Zhao Y, Shao D, Li J (2013) Efficient removal of cobalt from aqueous solution using β-cyclodextrin modified graphene oxide. RSC Adv 3:9514–9521. https://doi.org/10.1039/C3RA41434E
Wang Q, Li J, Chen C, Ren X, Hu J, Wang X (2011) Removal of cobalt from aqueous solution by magnetic multiwalled carbon nanotube/iron oxide composites. Chem Eng J 174:126–133. https://doi.org/10.1016/j.cej.2011.08.059
Park Y, Shin W, Choi S-J (2011) Sorptive removal of cobalt, strontium and cesium onto manganese and iron oxide-coated montmorillonite from groundwater. J Radioanal Nucl Chem 292:837–852. https://doi.org/10.1007/s10967-011-1527-7
Zhang L, Wei J, Zhao X, Li F, Jiang F, Zhang M, Cheng X (2016) Removal of strontium(II) and cobalt(II) from acidic solution by manganese antimonate. Chem Eng J 302:733–743. https://doi.org/10.1016/j.cej.2016.05.040
Al Abdullah J, Al Lafi AG, Al Masri W, Amin Y, Alnama T (2016) Adsorption of cesium, cobalt, and lead onto a synthetic nano manganese oxide: behavior and mechanism. Water Air Soil Pollut 227:241. https://doi.org/10.1007/s11270-016-2938-4
Triantafyllou S, Christodoulou E, Neou-Syngouna P (1999) Removal of nickel and cobalt from aqueous solutions by Na-activated bentonite. Clays Clay Miner 47:567–572. https://doi.org/10.1346/CCMN.1999.0470503
Manohar DM, Noeline BF, Anirudhan TS (2006) Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. Appl Clay Sci 31:194–206. https://doi.org/10.1016/j.clay.2005.08.008
Taka AL, Fosso-Kankeu E, Pillay K, Mbianda XY (2018) Removal of cobalt and lead ions from wastewater samples using an insoluble nanosponge biopolymer composite: adsorption isotherm, kinetic, thermodynamic, and regeneration studies. Environ Sci Pollut Res 25:21752–21767. https://doi.org/10.1007/s11356-018-2055-6
Zhu T, Tan S, Tong L, Wang Y, Wang R (2011) Characterization of a novel biopolymer by FT-IR spectroscopy and its use for cobalt removal. In: 2011 third international conference on measuring technology and mechatronics automation. pp 700–703
Vilvanathan S, Shanthakumar S (2017) Column adsorption studies on nickel and cobalt removal from aqueous solution using native and biochar form of Tectona grandis. Environ Prog Sustain Energy 36:1030–1038. https://doi.org/10.1002/ep.12567
Qasim HM, Abudi ZN, Alzubaidi LA (2022) Cobalt ion removal using magnetic biochar obtained from conocarpus erectus leaves. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02307-5
Lim Y, Kim B, Jang J, Lee DS (2022) Buckwheat hull-derived biochar immobilized in alginate beads for the adsorptive removal of cobalt from aqueous solutions. J Hazardous Mater 436:129245. https://doi.org/10.1016/j.jhazmat.2022.129245
Shin J, Kwak J, Lee Y-G, Kim S, Son C, Cho KH, Lee S-H, Park Y, Ren X, Chon K (2021) Changes in adsorption mechanisms of radioactive barium, cobalt, and strontium ions using spent coffee waste biochars via alkaline chemical activation: Enrichment effects of O-containing functional groups. Environ Res 199:111346. https://doi.org/10.1016/j.envres.2021.111346
Jiang J, Peng Y, Yuan M, Hong Z, Wang D, Xu R (2015) Rice straw-derived biochar properties and functions as Cu(II) and cyromazine sorbents as influenced by pyrolysis temperature. Pedosphere 25:781–789. https://doi.org/10.1016/S1002-0160(15)30059-X
Panwar NL, Pawar A (2022) Influence of activation conditions on the physicochemical properties of activated biochar: a review. Biomass Conv Bioref 12:925–947. https://doi.org/10.1007/s13399-020-00870-3
Yi Y, Huang Z, Lu B, Xian J, Tsang EP, Cheng W, Fang J, Fang Z (2020) Magnetic biochar for environmental remediation: a review. Bioresour Technol 298:122468. https://doi.org/10.1016/j.biortech.2019.122468
Zhao F, Repo E, Yin D, Sillanpää MET (2013) Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: Kinetics and isotherms. J Colloid Interface Sci 409:174–182. https://doi.org/10.1016/j.jcis.2013.07.062
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261. https://doi.org/10.1016/j.fuel.2017.12.054
Leng L, Huang H, Li H, Li J, Zhou W (2019) Biochar stability assessment methods: a review. Sci Total Environ 647:210–222. https://doi.org/10.1016/j.scitotenv.2018.07.402
Ahmed MB, Zhou JL, Ngo HH, Guo W (2016) Insight into biochar properties and its cost analysis. Biomass Bioenerg 84:76–86. https://doi.org/10.1016/j.biombioe.2015.11.002
Xu X, Wu Y, Wu X, Sun Y, Huang Z, Li H, Wu Z, Zhang X, Qin X, Zhang Y, Deng J, Huang J (2022) Effect of physicochemical properties of biochar from different feedstock on remediation of heavy metal contaminated soil in mining area. Surfaces Interfaces 32:102058. https://doi.org/10.1016/j.surfin.2022.102058
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Biores Technol 102:716–723. https://doi.org/10.1016/j.biortech.2010.08.067
Queiroz M, Melo K, Sabry D, Sassaki G, Rocha H (2014) Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs 13:141–158. https://doi.org/10.3390/md13010141
Lawrie G, Keen I, Drew B, Chandler-Temple A, Rintoul L, Fredericks P, Grøndahl L (2007) Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromol 8:2533–2541. https://doi.org/10.1021/bm070014y
Ren Y, Abbood HA, He F, Peng H, Huang K (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311. https://doi.org/10.1016/j.cej.2013.04.059
Qin R, Li F, Chen M, Jiang W (2009) Preparation of chitosan–ethylenediaminetetraacetate-enwrapped magnetic CoFe2O4 nanoparticles via zero-length emulsion crosslinking method. Appl Surf Sci 256:27–32. https://doi.org/10.1016/j.apsusc.2009.07.032
Tan Z, Peng H, Liu H, Wang L, Chen J, Lu X (2015) Facile preparation of EDTA-functionalized chitosan magnetic adsorbent for removal of Pb(II). J Appl Polymer Sci. https://doi.org/10.1002/app.42384
Lim S-F, Zheng Y-M, Zou S-W, Chen JP (2008) Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS, and mathematical modeling study. Environ Sci Technol 42:2551–2556. https://doi.org/10.1021/es7021889
Ma W, Ya F-Q, Han M, Wang R (2007) Characteristics of equilibrium, kinetics studies for adsorption of fluoride on magnetic-chitosan particle. J Hazard Mater 143:296–302. https://doi.org/10.1016/j.jhazmat.2006.09.032
Atia AA, Donia AM, Al-Amrani WA (2009) Adsorption/desorption behavior of acid orange 10 on magnetic silica modified with amine groups. Chem Eng J 150:55–62. https://doi.org/10.1016/j.cej.2008.12.004
Zhou S, Zheng X, Yu X, Wang J, Weng J, Li X, Feng B, Yin M (2007) Hydrogen bonding interaction of Poly(d, l-Lactide)/hydroxyapatite nanocomposites. Chem Mater 19:247–253. https://doi.org/10.1021/cm0619398
Graf N, Yegen E, Gross T, Lippitz A, Weigel W, Krakert S, Terfort A, Unger WES (2009) XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf Sci 603:2849–2860. https://doi.org/10.1016/j.susc.2009.07.029
Moradi P, Hajjami M (2022) Stabilization of ruthenium on biochar-nickel magnetic nanoparticles as a heterogeneous, practical, selective, and reusable nanocatalyst for the Suzuki C-C coupling reaction in water. RSC Adv 12:13523–13534. https://doi.org/10.1039/D1RA09350A
Khalil TE, Elhusseiny AF, Ibrahim NM, El-dissouky A (2021) Unexpected effect of magnetic nanoparticles on the performance of aqueous removal of toxic Cr(VI) using modified biopolymer chitosan. Int J Biol Macromol 170:768–779. https://doi.org/10.1016/j.ijbiomac.2020.12.188
Abdollahi B, Salari D, Zarei M (2022) Synthesis and characterization of magnetic Fe3O4@SiO2-MIL-53(Fe) metal-organic framework and its application for efficient removal of arsenate from surface and groundwater. J Environ Chem Eng 10:107144. https://doi.org/10.1016/j.jece.2022.107144
Langmuir I (1916) the constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(11):2221–2295
Freundlich H (1906) Over the adsorption in solution 57:1100–1107
Dada O, Olalekan A, Olatunya A, Dada AO (2012) Langmuir, freundlich, temkin and dubinin-radushkevich isotherms studies of equilibrium sorption of Zn 2+ Unto phosphoric acid modified rice husk. J Appl Chem 3:38–45. https://doi.org/10.9790/5736-0313845
Xu M, Zhang Y, Zhang Z, Shen Y, Zhao M, Pan G (2011) Study on the adsorption of Ca2+, Cd2+ and Pb2+ by magnetic Fe3O4 yeast treated with EDTA dianhydride. Chem Eng J 168:737–745. https://doi.org/10.1016/j.cej.2011.01.069
Thommes M, Cychosz KA (2014) Physical adsorption characterization of nanoporous materials: progress and challenges. Adsorption 20:233–250. https://doi.org/10.1007/s10450-014-9606-z
Şenol ZM (2021) A chitosan-based composite for adsorption of uranyl ions; mechanism, isothems, kinetics and thermodynamics. Int J Biol Macromol 183:1640–1648. https://doi.org/10.1016/j.ijbiomac.2021.05.130
Lin J, Wang L (2009) Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Eng China 3:320–324. https://doi.org/10.1007/s11783-009-0030-7
Zhang K, Dai Z, Zhang W, Gao Q, Dai Y, Xia F, Zhang X (2021) EDTA-based adsorbents for the removal of metal ions in wastewater. Coordinat Chem Rev 434:213809. https://doi.org/10.1016/j.ccr.2021.213809
Madadrang CJ, Kim HY, Gao G, Wang N, Zhu J, Feng H, Gorring M, Kasner ML, Hou S (2012) Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. ACS Appl Mater Interfaces 4:1186–1193. https://doi.org/10.1021/am201645g
Harikishore Kumar Reddy D, Lee S-M (2014) Magnetic biochar composite: facile synthesis, characterization, and application for heavy metal removal. Colloids Surf A 454:96–103. https://doi.org/10.1016/j.colsurfa.2014.03.105
Zhao J, Liang G, Zhang X, Cai X, Li R, Xie X, Wang Z (2019) Coating magnetic biochar with humic acid for high efficient removal of fluoroquinolone antibiotics in water. Sci Total Environ 688:1205–1215. https://doi.org/10.1016/j.scitotenv.2019.06.287
Kołodyńska D, Bąk J (2018) Use of three types of magnetic biochar in the removal of copper(II) ions from wastewaters. Sep Sci Technol 53:1045–1057. https://doi.org/10.1080/01496395.2017.1345944
Krishnan KA, Anirudhan TS (2008) Kinetic and equilibrium modelling of cobalt(II) adsorption onto bagasse pith based sulphurised activated carbon. Chem Eng J 137:257–264. https://doi.org/10.1016/j.cej.2007.04.029
Kragović M, Stojmenović M, Petrović J, Loredo J, Pašalić S, Nedeljković A, Ristović I (2019) Influence of alginate encapsulation on point of zero charge (pHpzc) and thermodynamic properties of the natural and Fe(III) - Modified Zeolite. Procedia Manuf 32:286–293. https://doi.org/10.1016/j.promfg.2019.02.216
Repo E, Koivula R, Harjula R, Sillanpää M (2013) Effect of EDTA and some other interfering species on the adsorption of Co(II) by EDTA-modified chitosan. Desalination 321:93–102. https://doi.org/10.1016/j.desal.2013.02.028
Foo KY, Hameed B (2011) An overview of dyes removal via activated carbon adsorption process. Desalin Water Treat 19:255–274
Huang Y, Wu H, Shao T, Zhao X, Peng H, Gong Y, Wan H (2018) Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: mechanism and application in simulated electroplating wastewater. Chem Eng J 339:322–333. https://doi.org/10.1016/j.cej.2018.01.071
Mei J, Mo S, Zhang H, Zheng X, Li Z (2020) Removal of Sr(II) from water with highly-elastic carboxymethyl chitosan gel. Int J Biol Macromol 163:1097–1105. https://doi.org/10.1016/j.ijbiomac.2020.07.038
Wang J-H, Atif S, Chen Y, Guo R (2020) Enhanced removal of Cu(II) from chemically diverse wastewater environment by EDTA-chitosan modified sewage sludge. DWT 190:167–178. https://doi.org/10.5004/dwt.2020.25647
Ebner AD, Ritter JA, Navratil JD (2001) Adsorption of cesium, strontium, and cobalt ions on magnetite and a magnetite−silica composite. Ind Eng Chem Res 40:1615–1623. https://doi.org/10.1021/ie000695c
El-Shamy OAA, El-Azabawy RE, El-Azabawy OE (2019) Synthesis and characterization of magnetite-alginate nanoparticles for enhancement of nickel and cobalt ion adsorption from wastewater. J Nanomater 2019:e6326012. https://doi.org/10.1155/2019/6326012
Medyńska-Juraszek A, Ćwieląg-Piasecka I, Jerzykiewicz M, Trynda J (2020) Wheat straw biochar as a specific sorbent of cobalt in soil. Materials 13:2462. https://doi.org/10.3390/ma13112462
Ahmadpour A, Tahmasbi M, Bastami TR, Besharati JA (2009) Rapid removal of cobalt ion from aqueous solutions by almond green hull. J Hazard Mater 166:925–930. https://doi.org/10.1016/j.jhazmat.2008.11.103
Pipíška M, Richveisová BM, Frišták V, Horník M, Remenárová L, Stiller R, Soja G (2017) Sorption separation of cobalt and cadmium by straw-derived biochar: a radiometric study. J Radioanal Nucl Chem 311:85–97. https://doi.org/10.1007/s10967-016-5043-7
Zhuang S, Yin Y, Wang J (2018) Removal of cobalt ions from aqueous solution using chitosan grafted with maleic acid by gamma radiation. Nucl Eng Technol 50:211–215. https://doi.org/10.1016/j.net.2017.11.007
Zhu Y, Hu J, Wang J (2014) Removal of Co2+ from radioactive wastewater by polyvinyl alcohol (PVA)/chitosan magnetic composite. Prog Nucl Energy 71:172–178. https://doi.org/10.1016/j.pnucene.2013.12.005
Zhuang S, Wang J (2019) Removal of cobalt ion from aqueous solution using magnetic graphene oxide/chitosan composite. Environ Prog Sustain Energy 38:S32–S41. https://doi.org/10.1002/ep.12912
Repo E, Warchol JK, Kurniawan TA, Sillanpää MET (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. Chem Eng J 161:73–82. https://doi.org/10.1016/j.cej.2010.04.030
Acknowledgements
This work was supported by the Nuclear Energy Development Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (2018M2B2B1065631).
Author information
Authors and Affiliations
Contributions
BP: Conceptualization, Methodology, Experiment, Investigation, Writing—original draft. SJC: Project administration, Supervision, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, B., Choi, SJ. Magnetic biochar modified with crosslinked chitosan and EDTA for removing cobalt from aqueous solutions. J Radioanal Nucl Chem 332, 2077–2091 (2023). https://doi.org/10.1007/s10967-023-08831-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08831-6