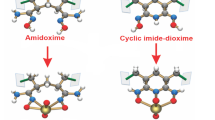
Abstract
Amidoxime is publicly acknowledged well-performed uranium adsorption material at present. However, its uranium adsorption is limited due to the influence of other metal ions. Protein has abundant amino and carboxyl and has high selectivity for a specific ion. Yet, it has not been used as an adsorbent only for seawater uranium extraction. Here, amidoxime and protein are combined to fabricate a composite film material (TB@PAO) to extract uranium from seawater. The simulated seawater experiment shows that the uranyl adsorption capacity of TB@PAO is 11.24 mg·g− 1, which is 4 times vanadium adsorption and 7 times iron adsorption. The uranyl’s adsorption distribution coefficient is 1.5 × 105, suggesting TB@PAO has high uranyl adsorption affinity. This work does not only provide an adsorbent for seawater uranium extraction, but also offer a way for the design of a highly selective seawater uranium extraction material.










Similar content being viewed by others
References
Shi S, Li B, Qian Y, Mei P, Wang N (2020) A simple and universal strategy to construct robust and anti-biofouling amidoxime aerogels for enhanced uranium extraction from seawater. Chem Eng J 397:125337
Abney CW, Mayes RT, Saito T, Dai S (2017) Materials for the recovery of uranium from seawater. Chem Rev 117(23):13935–14013
Zhang Z, Dong Z, Wang X, Ying D, Niu F, Cao X et al (2018) Ordered mesoporous polymer–carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chem Eng J 341:208–217
Luo W, Xiao G, Tian F, Richardson JJ, Wang Y, Zhou J et al (2019) Engineering robust metal–phenolic network membranes for uranium extraction from seawater. Energ Environ Sci 12(2):607–614
Wu F, Pu N, Ye G, Sun T, Wang Z, Song Y et al (2017) Performance and mechanism of uranium adsorption from seawater to poly(dopamine)-Inspired sorbents. Environ Sci Technol 51(8):4606–4614
Zänker H, Heine K, Weiss S, Brendler V, Husar R, Bernhard G et al (2019) Strong uranium(VI) binding onto bovine milk proteins, selected protein sequences, and model peptides. Inorg Chem 58(7):4173–4189
Han X, Wang Y, Cao X, Dai Y, Liu Y, Dong Z et al (2019) Adsorptive performance of ship-type nano-cage polyoxometalates for U(VI) in aqueous solution. Appl Surf Sci 484:1035–1040
Hirotsu T, Katoh S, Sugasaka K, Takai N, Seno M, Itagaki T (1987) Adsorption of uranium on cross-linked amidoxime polymer from seawater. Ind Eng Chem Res 26(10):1970–1977
Kavakli PA, Seko N, Tamada M, Güven O (2005) A highly efficient chelating polymer for the adsorption of Uranyl and Vanadyl ions at low concentrations. Adsorption 10(4):309–315
Yue Y, Mayes RT, Kim J, Fulvio PF, Sun X-G, Tsouris C et al (2013) Seawater uranium sorbents: preparation from a mesoporous copolymer initiator by atom-transfer radical polymerization. Angew Chem Int Ed 52(50):13458–13462
Lashley MA, Mehio N, Nugent JW, Holguin E, Do-Thanh C-L, Bryantsev VS et al (2016) Amidoximes as ligand functionalities for braided polymeric materials for the recovery of uranium from seawater. Polyhedron 109:81–91
Das S, Oyola Y, Mayes RT, Janke CJ, Kuo LJ, Gill G et al (2016) Extracting uranium from seawater: promising AI series adsorbents. Ind Eng Chem Res 55(15):4103–4109
Earl LD, Do C, Wang Y, Abney CW (2019) Polyamidoxime chain length drives emergent metal-binding phenomena. PCCP 21(2):554–560
Pan H-B, Wai CM, Kuo L-J, Gill GA, Wang JS, Joshi R et al (2020) A highly efficient uranium grabber derived from acrylic fiber for extracting uranium from seawater. Dalton T 49(9):2803–2810
Zhao S, Yuan Y, Yu Q, Niu B, Liao J, Guo Z et al (2019) A dual-surface Amidoximated halloysite nanotube for high-efficiency economical uranium extraction from seawater. Angew Chem Int Ed 58(42):14979–14985
Liu R, Wen S, Sun Y, Yan B, Wang J, Chen L et al (2021) A nanoclay enhanced Amidoxime-functionalized double-network hydrogel for fast and massive uranium recovery from seawater. Chem Eng J 422:130060
Kim J, Tsouris C, Oyola Y, Janke CJ, Mayes RT, Dai S et al (2014) Uptake of uranium from seawater by Amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment. Ind Eng Chem Res 53(14):6076–6083
Parker BF, Zhang Z, Leggett CJ, Arnold J, Rao L (2017) Kinetics of complexation of V(v), U(vI), and Fe(III) with glutaroimide-dioxime: studies by stopped-flow and conventional absorption spectroscopy. Dalton Trans 46(33):11084–11096
Das S, Brown S, Mayes RT, Janke CJ, Tsouris C, Kuo LJ et al (2016) Novel poly(imide dioxime) sorbents: development and testing for enhanced extraction of uranium from natural seawater. Chem Eng J 298:125–135
Wang D, Song J, Wen J, Yuan Y, Liu Z, Lin S et al (2018) Significantly enhanced uranium extraction from seawater with mass produced fully amidoximated nanofiber adsorbent. Adv Energy Mater 8(33):1802607
Gill GA, Kuo L-J, Janke CJ, Park J, Jeters RT, Bonheyo GT et al (2016) The uranium from seawater program at the Pacific Northwest National Laboratory: overview of marine testing, adsorbent characterization, adsorbent durability, adsorbent toxicity, and deployment studies. Ind Eng Chem Res 55(15):4264–4277
Ladshaw AP, Ivanov AS, Das S, Bryantsev VS, Tsouris C, Yiacoumi S (2018) First-principles Integrated adsorption modeling for selective capture of uranium from seawater by polyamidoxime sorbent materials. ACS Appl Mater Int 10(15):12580–12593
Seko N, Tamada M, Yoshii F (2005) Current status of adsorbent for metal ions with radiation grafting and crosslinking techniques. Nucl Instrum Methods B 236(1):21–29
Gomez-Maldonado D, Erramuspe IBV, Peresin MS (2019) Natural polymers as alternative adsorbents and treatment agents for Water remediation. BioResources 14:10093–10160
Zhou L, Bosscher M, Zhang C, Özçubukçu, Salih, Zhang L, Zhang W et al (2014) A protein engineered to bind uranyl selectively and with femtomolar affinity. Nat Chem 6(3):236
Odoh SO, Bondarevsky GD, Karpus J, Cui Q, He C, Spezia R et al (2014) UO22 + uptake by proteins: understanding the binding features of the Super Uranyl-Binding protein (SUP) and design of a protein with higher Affinity. J Am Chem Soc 136(50):17484–17494
Li W, Liu Q, Liu J, Zhang H, Li R, Li Z et al (2017) Removal U(VI) from artificial seawater using facilely and covalently grafted polyacrylonitrile fibers with lysine. Appl Surf Sci 403:378–388
Tian G, Teat SJ, Rao L (2013) Thermodynamic studies of U(vi) complexation with glutardiamidoxime for sequestration of uranium from seawater. Dalton Trans 42(16):5690–5696
Li W, Liu Y-Y, Bai Y, Wang J, Pang H (2020) Anchoring ZIF-67 particles on amidoximerized polyacrylonitrile fibers for radionuclide sequestration in wastewater and seawater. J Hazard Mater 395:122692
Wang F, Li H, Liu Q, Li Z, Li R, Zhang H et al (2016) A graphene oxide/amidoxime hydrogel for enhanced uranium capture. Sci Rep-UK 6(1):19367
Yang J, Volesky B (1999) Biosorption of uranium on sargassum biomass. Water Res 33(15):3357–3363
Wang H, Yao H, Chen L, Yu Z, Yang L, Li C et al (2020) Highly efficient capture of uranium from seawater by layered double hydroxide composite with benzamidoxime. Sci Total Environ 759:143483
Panias D, Krestou A (2004) Uranium (VI) speciation diagrams in the UO22+/CO32-/H2O system at 25°C. Eur J Mineral Process Environ Prot 4:113–129
Zuo L, Peng W, Xu Z, Guo H, Luo M (2022) Selective adsorption of uranyl by glutamic acid-modified amidoxime fiber. React Funct Polym 179:105376
Wegner SV, Boyaci H, Chen H, Jensen MP, He C (2009) Engineering A Uranyl-specific binding protein from NikR. Angew Chem Int Ed 48(13):2339–2341
Bolotin DS, Bokach NA, Kukushkin VY (2016) Coordination chemistry and metal-involving reactions of amidoximes: relevance to the chemistry of oximes and oxime ligands. Coord Chem Rev 313:62–93
Kuo L-J, Janke CJ, Wood JR, Strivens JE, Das S, Oyola Y et al (2016) Characterization and testing of Amidoxime-based adsorbent materials to extract uranium from natural seawater. Ind Eng Chem Res 55(15):4285–4293
Xie S, Liu X, Zhang B, Ma H, Ling C, Yu M et al (2015) Electrospun nanofibrous adsorbents for uranium extraction from seawater. J Mater Chem A 3(6):2552–2558
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2016) Gaussian 16. Revision A, 3rd edn. Gaussian, Wallingford
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 22065002), The State Key Laboratory for Nuclear Resources and Environment, (East China Institute of Technology) (Nos. NRE2021-16, 2020NRE31).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuo, L., Guo, H., Xu, Z. et al. Trypsin-modified amidoxime improves the adsorption selectivity of uranium. J Radioanal Nucl Chem 332, 713–722 (2023). https://doi.org/10.1007/s10967-023-08770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08770-2