Abstract
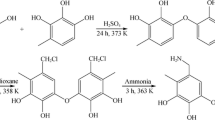
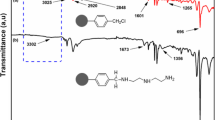
Developing the recovery technology of rhenium under the background of high molybdenum is of great significance to the recovery of rhenium resources in the leaching solution of molybdenum concentrate roasting. Herein, the resin LSL-N with adsorption capacity for rhenium and molybdenum was synthesized with N-methylimidazole as the functional group. The results of the batch adsorption test show that LSL-N resin has a good adsorption capacity for Re(VII) and Mo(VI), and the saturated adsorption capacity is 342.26 mg/g and 185.06 mg/g. The existing form of molybdenum (MO42–) in the solution was calculated by PHREEQC. The shorter hydrogen bond distance of MO42–@2LSL-N indicates that LSL-N resin has a stronger adsorption capacity for Mo(VI) when the active sites are sufficient, which is revealed by density functional theory (DFT) calculation. LSL-N resin can enrich Re(VII) and Mo(VI) in the eluent at the same time, with the maximum enrichment factors of 134.8 and 38.6. Re(VII) and Mo(VI) can be desorbed step by step with different desorbents, and molybdenum can be recovered as well as rhenium.















Similar content being viewed by others
References
Liu H (2019) Study on the escape of rhenium in the roasting process of molybdenite and the adsorption mechanism of leaching solution. General Research Institute for Nonferrous Metals, Beijing
Hong T, Liu M, Ma J, Yang G, Li L, Mumford KA, Stevens GW (2020) Selective recovery of Rhenium from industrial leach solutions by synergistic solvent extraction. Sep Purif Technol 236:116281
Fathi MB, Rezai B, Alamdari E, Alorro RD (2017) Mechanism and equilibrium modeling of Re and Mo adsorption on a gel type strong base anion resin. Russ J Appl Chem 90:1504–1513
Anderson CD, Taylor PR, Anderson CG (2013) Extractive metallurgy of rhenium: a review. Min Metall Explor 30(1):59–73
Xian W, Li L, Zhang W, Zhang X (2008) Properties of rhenium and distribution of rhenium resources. Express Inf Min Ind 11(11):67–69
Liao R, Liu H, Li C, Sun W (2020) The prospecting prospect of rhenium in China from the geochemical properties of rhenium. Acta Petrol Sin 36(01):55–67. https://doi.org/10.18654/1000-0569/2020.01.07
Huang Y, Zhang B, Liu B et al (2021) Clean and deep separation of molybdenum and rhenium from ultra-low concentration solutions via vapidly stepwise selective coagulation and flocculation precipitation. Sep Purif Technol 267(8):118632
Wang P (2019) Enrichment of trace Pt(IV), Pd(II), and Au(III) in aqueous solution by imidazole-based polymer ionic liquid. Lanzhou University, Lanzhou
Che R (2017) Preparation of cobalt-based zeolite imidazole ester framework material and its application in simulated seawater uranium extraction. Harbin Engineering University, Harbin
Lou Z, Xing S, Xiao X, Shan W, Xiong Y, Fan Y (2018) Selective adsorption of Re(VII) by chitosan modified with imidazolium-based ionic liquid. Hydrometallurgy 179:141–148. https://doi.org/10.1016/j.hydromet.2018.05.025
Zou Y (2017) Study on adsorption and mechanism of radionuclide uranium by functional hydrotalcite (LDHs-X) clay minerals. East China University of Technology, Nanchang
Zhang W, Ma J, Liu S, Lipeng Wu, Xie H, Wen J (2014) Adsorption of uranium on modified graphene sponge. J East China Univ Technol (Nat Sci) 37(02):230–235
Zagorodnyaya AN, Abisheva ZS, Sharipova AS, Sadykanova SE, Bochevskaya YG, Atanova OV (2013) Sorption of rhenium and uranium by strong base anion exchange resin from solutions with different anion compositions. Hydrometallurgy 131–132:127–132. https://doi.org/10.1016/j.hydromet.2012.11.003
Ye M (2015) Synthesis of anion exchange resin and its adsorption and desorption behavior for rhenium and technetium. Shanghai Jiao Tong University, Shanghai
Han D, Li X, Peng J, Ling Xu, Li J, Li H (2016) A new imidazolium-based polymeric ionic liquid gel with high adsorption capacity for perrhenate. RSC Adv 6(73):69052–69059
Liu H, Zhang Bo, Jing X, Wang W, Wang L (2018) Adsorption and desorption properties for rhenium using a kind of weak-base anion resin. Rare Met 37(08):707–715
Miniakhmetov IA, Semenov SA, Musatova VY, Reznik AM (2013) Solvent extraction of rhenium with N-(2-hydroxy-5-nonylbenzyl)-β-hydroxyethylmethylamine. Russ J Inorg Chem 58(11):1380–1382
Rivas BL, Espinosa C, Sánchez J (2018) Application of the liquid-phase polymer-based retention technique to the sorption of molybdenum(VI) and vanadium(V). Polym Bull. https://doi.org/10.1007/s00289-018-2397-8
Shan W, Shu Y, Chen H, Zhang D, Wang W, Hongqiang Ru, Xiong Y (2016) The recovery of molybdenum(VI) from rhenium(VII) on amino-functionalized mesoporous materials. Hydrometallurgy 165:251–260. https://doi.org/10.1016/j.hydromet.2016.02.005
Zhang Z, Zhou R, Dong Z, Cao X, Liu Y (2020) Adsorption of U(VI)-CO3/Ca-U(VI)-CO3 by amidoxime hydrothermal carbon. J Inorg Mater 35(03):352–358
Acknowledgements
This project was mainly supported by the Nuclear Energy Development Project (technology for the mining and metallurgy of associated uranium resources—on the demonstration of uranium co-mining in Bayan Ura, Inner Mongolia) and China Uranium Industry Co., Ltd.—the Foundation of State Key Laboratory of Nuclear Resources and Environment Joint Innovation Fund Project (2022NRE-LH-15).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, J., Li, J., Liao, Y. et al. Rhenium recovery from roasting leachate of molybdenum concentrate by N-methylimidazole functionalized anion exchange resin. J Radioanal Nucl Chem 332, 747–760 (2023). https://doi.org/10.1007/s10967-022-08755-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08755-7