Abstract
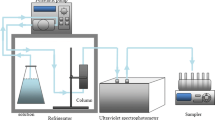
Since little is known about the colloidal Layered double hydroxides (LDH) stability and how colloidal LDH influences U(VI) transport, column experiments were performed to study the co-transport of U(VI) and MgAl-LDH colloids in water-saturated granite particle as a function of important environmental factors. It was found that the stability and transport of U(VI)-bearing LDH colloid was affacted by colloid concentration, ionic strength and pH. A two-site kinetic attachment/detachment model was applied to describe the breakthrough curves of U(VI)-bearing LDH colloid. The experimental and modeling results of this study imply that a risk assessment of LDH colloid facilitated transport should be considered.






Similar content being viewed by others
References
Chen S, Wei X, Liu J, Sun Z, Chen G, Yang M, Liu Y, Wang D, Ma C, Kong D (2022) Weak acid leaching of uranium ore from a high carbonate uranium deposit. J Radioanal Nucl Chem 331:2583–2596. https://doi.org/10.1007/s10967-022-08323-z
Kacham AR, Suri AK (2014) Application of a topochemical reaction model to predict leaching behavior of high carbonate uranium ores in alkaline solutions: an experimental case study. Hydrometallurgy 141:67–75. https://doi.org/10.1016/j.hydromet.2013.10.005
Smirnov KM, Molchanova TV, Akimova ID, Krylova OK (2018) Efficient technology for combined processing of silicate and carbonate uranium ores. At Energy 124:111–117. https://doi.org/10.1007/s10512-018-0383-8
Zhang X, Ji L, Wang J, Li R, Liu Q, Zhang M, Liu L (2012) Removal of uranium(VI) from aqueous solutions by magnetic Mg–Al layered double hydroxide intercalated with citrate: kinetic and thermodynamic investigation. Colloids Surf A Physicochem Eng Asp 414:220–227. https://doi.org/10.1016/j.colsurfa.2012.08.031
Yang Y, Saiers JE, Xu N, Minasian SG, Tyliszczak T, Kozimor SA, Shuh DK, Barnett MO (2012) Impact of natural organic matter on uranium transport through saturated geologic materials: from molecular to column scale. Environ Sci Technol 46:5931–5938. https://doi.org/10.1021/es300155j
Chen C, Zhao K, Shang J, Liu C, Wang J, Yan Z, Liu K, Wu W (2018) Uranium (VI) transport in saturated heterogeneous media: influence of kaolinite and humic acid. Environ Pollut 240:219–226. https://doi.org/10.1016/j.envpol.2018.04.095
Wang Q, Cheng T, Wu Y (2014) Influence of mineral colloids and humic substances on uranium(VI) transport in water-saturated geologic porous media. J Contam Hydrol 170:76–85. https://doi.org/10.1016/j.jconhyd.2014.10.007
Niu Z, Wei X, Qiang S, Wu H, Pan D, Wu W, Fan Q (2019) Spectroscopic studies on U(VI) incorporation into CaCO3: effects of aging time and U(VI) concentration. Chemosphere 220:1100–1107. https://doi.org/10.1016/j.chemosphere.2019.01.010
IAEA (2004) Treatment of liquid effluent from uranium mines and mills. Report of a co-ordinated research project 1996–2000. International Atomic Energy Agency, Vienna, pp 95–102
Yang J, Zhang Z, Chen Z, Ge M, Wu W, Guo Z (2019) Co-transport of U(VI) and gibbsite colloid in saturated granite particle column: role of pH, U(VI) concentration and humic acid. Sci Total Environ 688:450–461. https://doi.org/10.1016/j.scitotenv.2019.05.395
Möri A, Alexander WR, Geckeis H, Hauser W, Schäfer T (2003) The colloid and radionuclide retardation experiment at the Grimsel Test Site: influence of bentonite colloids on radionuclide migration in a fractured rock. Colloids Surf A Physicochem Eng Asp 217:33–47. https://doi.org/10.1016/S0927-7757(02)00556-3
Ge M, Wang D, Yang J, Jin Q, Chen Z, Wu W, Guo Z (2018) Co-transport of U(VI) and akaganeite colloids in water-saturated porous media: role of U(VI) concentration, pH and ionic strength. Water Res 147:350–361. https://doi.org/10.1016/j.watres.2018.10.004
Zhang Z, Heng J, Jin Q, Chen Z, Wu W, Guo Z (2022) Co-transport of bentonite colloid and U(VI) in particulate granite column: role of colloid concentration, ionic strength, pH and flow rate. Radiochim Acta. https://doi.org/10.1515/ract-2021-1096
Sun Y, Zhang Z, Heng J, Jin Q, Chen Z, Guo Z (2022) Co-transport of U(VI) and colloidal biochar in quartz sand heterogeneous media. Sci Total Environ 816:151606. https://doi.org/10.1016/j.scitotenv.2021.151606
Wang D, Paradelo M, Bradford SA, Peijnenburg WJ, Chu L, Zhou D (2011) Facilitated transport of Cu with hydroxyapatite nanoparticles in saturated sand: effects of solution ionic strength and composition. Water Res 45:5905–5915. https://doi.org/10.1016/j.watres.2011.08.041
Liao L, Zhao N, Xia Z (2012) Hydrothermal synthesis of Mg–Al layered double hydroxides (LDHs) from natural brucite and Al(OH)3. Mater Res Bull 47:3897–3901. https://doi.org/10.1016/j.materresbull.2012.07.007
Li C, Wei Y, Wang X, Yin X (2018) Efficient and rapid adsorption of iodide ion from aqueous solution by porous silica spheres loaded with calcined Mg-Al layered double hydroxide. J Taiwan Inst Chem Eng 85:193–200. https://doi.org/10.1016/j.jtice.2018.01.044
Kasel D, Bradford SA, Simunek J, Heggen M, Vereecken H, Klumpp E (2013) Transport and retention of multi-walled carbon nanotubes in saturated porous media: effects of input concentration and grain size. Water Res 47:933–944. https://doi.org/10.1016/j.watres.2012.11.019
Jiang X, Zhang M, Yan B, Hu J, Chen J, Guan Y (2019) Roles of Mg-Al layered double hydroxides and solution chemistry on P transport in soil. Chem Eng J 373:1111–1119. https://doi.org/10.1016/j.cej.2019.05.083
Rahman MT, Kameda T, Kumagai S, Yoshioka T (2016) Adsorption isotherms and kinetics of arsenic removal from aqueous solution by Mg–Al layered double hydroxide intercalated with nitrate ions. React Kinet Mech Catal 120:703–714. https://doi.org/10.1007/s11144-016-1116-4
Zhang X, Zhang M, Li R, Feng X, Pang X, Rao J, Cong D, Yin C, Zhang Y (2021) Active corrosion protection of Mg–Al layered double hydroxide for magnesium alloys: a short review. Coatings. https://doi.org/10.3390/coatings11111316
Paikaray S, Gomez MA, Jim Hendry M, Essilfie-Dughan J (2014) Formation mechanism of layered double hydroxides in Mg2+-, Al3+-, and Fe3+-rich aqueous media: implications for neutralization in acid leach ore milling. Appl Clay Sci 101:579–590. https://doi.org/10.1016/j.clay.2014.09.022
Kasatkin AV, Britvin SN, Krzhizhanovskaya MG (2022) Kaznakhtite, Ni6Co3+2(CO3)(OH)16⋅4H2O, a new natural layered double hydroxide, the member of the hydrotalcite supergroup. Mineral Mag 86:841–848. https://doi.org/10.1180/mgm.2022.65
Paikaray S, Essilfie-Dughan J, Hendry MJ (2018) Ionic substitution of Mg2+ for Al3+ and Fe3+ with octahedral coordination in hydroxides facilitate precipitation of layered double hydroxides. Geochim Cosmochim Acta 220:217–234. https://doi.org/10.1016/j.gca.2017.10.003
Bouali AC, Serdechnova M, Blawert C, Tedim J, Ferreira MGS, Zheludkevich ML (2020) Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: a review. Appl Mater Today. https://doi.org/10.1016/j.apmt.2020.100857
Huo J, Min X, Dong Q, Xu S, Wang Y (2022) Comparison of Zn-Al and Mg-Al layered double hydroxides for adsorption of perfluorooctanoic acid. Chemosphere 287:132297. https://doi.org/10.1016/j.chemosphere.2021.132297
Benhiti R, Ait Ichou A, Zaghloul A, Aziam R, Carja G, Zerbet M, Sinan F, Chiban M (2020) Synthesis, characterization, and comparative study of MgAl-LDHs prepared by standard coprecipitation and urea hydrolysis methods for phosphate removal. Environ Sci Pollut Res Int 27:45767–45774. https://doi.org/10.1007/s11356-020-10444-5
Ye H (2021) Autogenous formation and smart behaviors of nitrite- and nitrate-intercalated layered double hydroxides (LDHs) in Portland cement-metakaolin-dolomite blends. Cem Concr Res. https://doi.org/10.1016/j.cemconres.2020.106267
Yin Q, Lyu P, Wang G, Wang B, Li Y, Zhou Z, Guo Y, Li L, Deng N (2022) Phosphorus-modified biochar cross-linked Mg-Al layered double-hydroxide stabilizer reduced U and Pb uptake by Indian mustard (Brassica juncea L.) in uranium contaminated soil. Ecotoxicol Environ Saf 234:113363. https://doi.org/10.1016/j.ecoenv.2022.113363
Yin L, Hu Y, Ma R, Wen T, Wang X, Hu B, Yu Z, Hayat T, Alsaedi A, Wang X (2019) Smart construction of mesoporous carbon templated hierarchical Mg-Al and Ni-Al layered double hydroxides for remarkably enhanced U(VI) management. Chem Eng J 359:1550–1562. https://doi.org/10.1016/j.cej.2018.11.017
Jana A, Unni A, Ravuru SS, Das A, Das D, Biswas S, Sheshadri H, De S (2022) In-situ polymerization into the basal spacing of LDH for selective and enhanced uranium adsorption: a case study with real life uranium alkaline leach liquor. Chem Eng J. https://doi.org/10.1016/j.cej.2021.131180
Zhang Z, Gao P, Montavon G, Chen Z, Wang D, Tan Z, Jin Q, Wu W, Wang J, Guo Z (2022) Strengthened erosion resistance of compacted bentonite by layered double hydroxide: a new electrostatic interaction-based approach. Chemosphere 292:133402. https://doi.org/10.1016/j.chemosphere.2021.133402
Liu Y (2005) Synthesis and application of layered double hydroxides. Chem Ind Times 19:59–62. https://doi.org/10.16597/j.cnki.issn.1002-154x.2005.12.019
Cao Y, Zheng D, Zhang F, Pan J, Lin C (2022) Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: a review. J Mater Sci Technol 102:232–263. https://doi.org/10.1016/j.jmst.2021.05.078
Zolgharnein J, Jerdi AS, Azimi GG, Jahanbakhsh (2009) Spectrophotometric determination of trace amounts of fluoride using an Al-xylenol orange complex as a colored reagent. Anal Sci 25:1249–1253
Guo Z, Li Y, Wu W (2009) Sorption of U(VI) on goethite: effects of pH, ionic strength, phosphate, carbonate and fulvic acid. Appl Radiat Isot 67:996–1000. https://doi.org/10.1016/j.apradiso.2009.02.001
Šimůnek J, Genuchten MT (2008) Modeling nonequilibrium flow and transport processes using HYDRUS. Vadose Zone J 7:782–797. https://doi.org/10.2136/vzj2007.0074
Liu Q, Xu S, Liu J (2008) Comparison between kaolinite and SiO2 colloidin transport behavior in saturated porous media. Acta Pedol Sin 45:446–451
Sun H, Wang Y (2010) Study on effect of clay mineral colloid on the environmental behavior of Pb. Northwest A&F Universit
Kretzschmar R, Borkovec M, Grolimund D, Elimelech M (1999) Mobile subsurface colloids and their role in contaminant transport. In: Advances in agronomy, vol 66, pp 121–193. https://doi.org/10.1016/s0065-2113(08)60427-7
Cai L, Tong M, Ma H, Kim H (2013) Cotransport of titanium dioxide and fullerene nanoparticles in saturated porous media. Environ Sci Technol 47:5703–5710. https://doi.org/10.1021/es400256d
Yang J, Guo Z, Chen Z (2019) The effects of gibbsite colloids and humic acid on U(VI) transport through saturated porous media. Lanzhou University
Dittrich TM, Boukhalfa H, Ware SD, Reimus PW (2015) Laboratory investigation of the role of desorption kinetics on americium transport associated with bentonite colloids. J Environ Radioact 148:170–182. https://doi.org/10.1016/j.jenvrad.2015.07.001
Nguyen THD, Zhang Z, Mustapha A, Li H, Lin M (2014) Use of graphene and gold nanorods as substrates for the detection of pesticides by surface enhanced Raman spectroscopy. J Agric Food Chem 62:10445–10451. https://doi.org/10.1021/jf5036417
Torkzaban S, Bradford SA, Vanderzalm JL, Patterson BM, Harris B, Prommer H (2015) Colloid release and clogging in porous media: effects of solution ionic strength and flow velocity. J Contam Hydrol 181:161–171. https://doi.org/10.1016/j.jconhyd.2015.06.005
Torkzaban SB, Walker SA, Sharon L (2007) Resolving the coupled effects of hydrodynamics and DLVO forces on colloid attachment in porous media. Am Chem Soc 23:9625–9660. https://doi.org/10.1021/la700995e
Jiang Y, Lin G (2013) Migration-deposition of Nano-hydroxyapatite and induced removal of Manganese in Ground water. Ocean University of China
Simunek JS, Saito M, Sakai H, van Genuchten MT (2008) The HYDRUS-1D software package for simulating the one-dimensional movement of water, heat, and multiple solutes in variably-saturated media. Department of Environmental Science, vol Version 4.16. University of California Riverside, Riverside, California
Wang D, Su C, Zhang W, Hao X, Cang L, Wang Y, Zhou D (2014) Laboratory assessment of the mobility of water-dispersed engineered nanoparticles in a red soil (Ultisol). J Hydrol 519:1677–1687. https://doi.org/10.1016/j.jhydrol.2014.09.053
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant Nos. 22176079, 21806064, 21906074), the Natural Science Foundation of Gansu Province, China (Nos. 22JR5RA480, 21JR7RA513), and Fundamental Research Funds for the Central Universities (lzujbky-2021-32, lzujbky-2022-sp05).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Hou, H., Gao, G. et al. Co-transport of colloidal MgAl-LDH and U(VI) in saturated granite particle column: role of colloid concentration, ionic strength, pH and flow rate. J Radioanal Nucl Chem 332, 1181–1191 (2023). https://doi.org/10.1007/s10967-022-08737-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08737-9