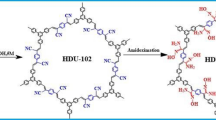
Abstract
Developing novel adsorbents for efficient U(VI) adsorption from wastewater is of great significance in both nuclear energy development and environmental protection. Herein, a novel functional resin (AO67/MA33-resin) with large particle size (100–200 μm) was controllably synthesized and employed for U(VI) elimination. The AO67/MA33-resin overcomes the shortcoming of small particle size of traditional adsorbents and shows strong antacid and high-temperature resistance stability and reusability. Meanwhile, the AO67/MA33-resin also exhibits excellent U(VI) removal ability including wide pH adaptability, fast adsorption kinetic, and high adsorption capacity. The adsorption mechanism was determined to involve chelation between amidoxime carboxyl functional groups and uranyl ions. A further application for U(VI) removal from real uranium mine wastewater was evaluated by dynamic column experiments. The present work indicated that the AO67/MA33-resin would be a promising adsorbent material for uranium removal from wastewater.









Similar content being viewed by others
References
Brook BW, Alonso A, Meneley DA, Misak J, Blees T, van Erp JB (2014) Why nuclear energy is sustainable and has to be part of the energy mix. Sustain Mater Techno 1–2:8–16
Go AW, Conag AT, Igdon RMB, Toledo AS, Malila JS (2019) Potentials of agricultural and agro-industrial crop residues for the displacement of fossil fuels: a Philippine context. Energy Strateg Rev 23:100–113
Qureshi MI, Rasli AM, Zaman K (2016) Energy crisis, greenhouse gas emissions and sectoral growth reforms: repairing the fabricated mosaic. J Clean Prod 112(5):3657–3666
Feng LD (2021) Research on nuclear energy and fossil fuels in China. IOP Conf Ser Earth: Environ Sci 621:012068
Desgranges L, Ma Y, Garcia P, Baldinozzi G, Siméone D, Fischer HE (2017) What is the actual local crystalline structure of uranium dioxide, UO2? A new perspective for the most used nuclear fuel. Inorg Chem 56(1):321–326
Baker RJ (2014) Uranium minerals and their relevance to long term storage of nuclear fuels. Coordin Chem Rev 266–267:123–136
Gao N, Huang ZW, Liu HQ, Hou J, Liu XH (2019) Advances on the toxicity of uranium to different organisms. Chemosphere 237:124548
Wu Y, Wang YX, Xie XJ (2014) Occurrence, behavior and distribution of high levels of uranium in shallow groundwater at Datong basin, northern China. Sci Total Environ 472:809–817
World Health Organization (2004) Guidelines for drinking-water quality, vol 1. World health organization
Cheng YX, He P, Dong FQ, Nie XQ, Ding CC, Wang S, Zhang Y, Liu HH, Zhou SP (2019) Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem Eng J 367:198–207
Luo WS, Kelly SD, Kemner KM, Watson D, Zhou JZ, Jardine PM, Gu BH (2009) Sequestering uranium and technetium through co-precipitation with aluminum in a contaminated acidic environment. Environ Sci Technol 43(19):7516–7522
Kabay N, Demircioǧlu M, Yaylı S, Günay E, Yüksel M, Saǧlam M, Streat M (1998) Recovery of uranium from phosphoric acid solutions using chelating ion-exchange resins. Ind Eng Chem Res 37:1983–1990
Li ZJ, Huang ZW, Guo WL, Wang L, Zheng LR, Chai ZF, Shi WQ (2017) Enhanced photocatalytic removal of uranium(vi) from aqueous solution by magnetic TiO2 /Fe3O4 and its graphene composite. Environ Sci Technol 51(10):5666–5674
Li WT, Liu YY, Bai Y, Wang J, Pang H (2020) Anchoring ZIF-67 particles on amidoximerized polyacrylonitrile fibers for radionuclide sequestration in wastewater and seawater. J Hazard Mater 395:122692
Mishima K, Du XY, Miyamoto N, Kano N, Imaizumi H (2018) Experimental and theoretical studies on the adsorption mechanisms of uranium (VI) ions on chitosan. J Funct Biomater 9(3):49
Satpathy A, Catalano JG, Giammar DE (2022) Reduction of U(VI) on chemically reduced montmorillonite and surface complexation modeling of adsorbed U(IV). Environ Sci Technol 56(7):4111–4120
Liu P, Wu HY, Yuan N, Liu YQ, Pan DQ, Wu WS (2017) Removal of U(VI) from aqueous solution using synthesized β-zeolite and its ethylenediamine derivative. J Mol Liq 234:40–48
Zhu KR, Chen CL, Xu MWC, Chen K, Tan XL, Wakeel M, Alharbi NS (2018) In situ carbothermal reduction synthesis of Fe nanocrystals embedded into N-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction. Chem Eng J 331:395–405
Zhang PC, Wang L, Du K, Wang SY, Huang ZW, Yuan LY, Li ZJ, Wang HQ, Zheng LR, Chai ZF, Shi WQ (2020) Effective removal of U(VI) and Eu(III) by carboxyl functionalized MXene nanosheets. J Hazard Mater 396:122731
Liao J, Liu P, Xie Y, Zhang Y (2021) Metal oxide aerogels: Preparation and application for the uranium removal from aqueous solution. Sci Total Environ 768:144212
Xiao J, Chen YT, Xu JB (2014) Plasma grafting montmorillonite/iron oxide composite with β-cyclodextrin and its application for high-efficient decontamination of U(VI). J Ind Eng Chem 20(5):2830–2839
Luo BC, Yuan LY, Chai ZF, Shi WQ, Tang Q (2016) U(VI) capture from aqueous solution by highly porous and stable MOFs: UiO-66 and its amine derivative. J Radioanal Nucl Chem 307:269–276
Tao XQ, Yao XB, Lu SS, Wang MM (2015) Efficient removal of radionuclide U(VI) from aqueous solutions by using graphene oxide nanosheets. J Radioanal Nucl Chem 303:245–253
Chen M, Liu T, Zhang X, Zhang R, Tang S, Yuan Y, Xie Z, Liu Y, Wang H, Fedorovich KV, Wang N (2021) Photoinduced enhancement of uranium extraction from seawater by MOF/Black phosphorus quantum dots heterojunction anchored on cellulose nanofiber aerogel. Adv Funct Mater 31:2100106
Cui HM, Feng XJ, Shi JS, Liu WG, Yan NF, Rao GH, Wang W (2020) A facile process for enhanced rare earth elements separation from dilute solutions using N, N-di(2-ethylhexyl)-diglycolamide grafted polymer resin. Sep Purif Technol 234:116096
Sadeek SA, El-Sayed MA, Amine MM, Abd El-Magied MO (2013) A chelating resin containing trihydroxybenzoic acid as the functional group: synthesis and adsorption behavior for Th(IV) and U(VI) ions. J Radioanal Nucl Chem 299:1299–1306
Wang MJ, Liu HZ, Zeng MQ, Liu YC (2022) Preparation of PAMAM dendrimer modified amidoxime chelating resin and its adsorption for U(VI) in aqueous. Inorg Chem Commun 144:109909
Dai Y, Jin JY, Zhou LM, Li TQ, Li Z, Liu ZR, Huang GL, Adesina AA (2016) Preparation of hollow SiO2 microspheres functionalized with amidoxime groups for highly efficient adsorption of U(VI) from aqueous solution. J Radioanal Nucl Chem 311:2029–2203
Liu JM, Liu T, Wang CC, Yin XH, Xiong ZH (2017) Introduction of amidoxime groups into metal-organic frameworks to synthesize MIL-53(Al)-AO for enhanced U(VI) sorption. J Mol Liq 242:531–536
Shao DD, Wang XL, Ren XM, Hu S, Wen J, Tan ZY, Xiong J, Asiri AM, Marwani HM (2018) Polyamidoxime functionalized with phosphate groups by plasma technique for effective U(VI) adsorption. J Ind Eng Chem 67:380–387
Zhang B, Guo XJ, Xie SY, Liu XY, Ling CJ, Ma HJ, Yu M, Li JY (2016) Synergistic nanofibrous adsorbent for uranium extraction from seawater. RSC Adv 6:81995–82005
Ahmad M, Wu F, Cui YH, Zhang QY, Zhang BL (2020) Preparation of novel bifunctional magnetic tubular nanofibers and their application in efficient and irreversible uranium trap from aqueous solution. ACS Sustain Chem Eng 8:7825–7838
Da’na E (2017) Adsorption of heavy metals on functionalized-mesoporous silica: a review. Micropor Mesopor Mater 247:145–157
Zhao R, Shi XY, Ma TT, Rong HZ, Wang ZY, Cui FC, Zhu GS, Wang C (2021) Constructing mesoporous adsorption channels and MOF-polymer interfaces in electrospun composite fibers for effective removal of emerging organic contaminants. ACS Appl Mater Interf 13:755–764
Wen R, Li Y, Zhang MC, Guo XH, Li X, Li XF, Han J, Hu S, Tan W, Ma LJ, Li SJ (2018) Graphene-synergized 2D covalent organic framework for adsorption: a mutual promotion strategy to achieve stabilization and functionalization simultaneously. J Hazard Mater 358:273–285
Govindarajan M, Karabacak M, Suvitha A, Periandy S (2012) FT-IR, FT-raman, ab initio, HF and DFT studies, NBO, HOMO-LUMO and electronic structure calculations on 4-chloro-3-nitrotoluene. Spectrochim Acta A Mol Biomol Spectrosc 89:137–148
Gao QH, Hu JT, Li R, Xing Z, Xu L, Wang MH, Guo XJ, Wu GZ (2016) Radiation synthesis of a new amidoximated UHMWPE fibrous adsorbent with high adsorption selectivity for uranium over vanadium in simulated seawater. Radiat Phys Chem 122:1–8
Groetsch T, Maghe M, Rana R, Hess R, Nunna S, Herron J, Buckmaster D, Creighton C, Varley RJ (2021) Gas emission study of the polyacrylonitrile-based continuous pilot-scale carbon fiber manufacturing process. Ind Eng Chem Res 60:17379–17389
Fu MT, Ao JX, Ma L, Kong DX, Qi SM, Zhang P, Xu G, Wu MH, Ma HJ (2022) Uranium removal from waste water of the tailings with functional recycled plastic membrane. Sep Purif Technol 287:120572
Zheng H, Zhou L, Liu Z, Le Z, Ouyang J, Huang G, Shehzad H (2019) Functionalization of mesoporous Fe3O4@SiO2 nanospheres for highly efficient U(VI) adsorption. Micropor Mesopor Mater 279:316–322
Yuan F, Wu CF, Cai YW, Zhang LJ, Wang JQ, Chen LH, Wang XK, Yang ST, Wang SA (2017) Synthesis of phytic acid-decorated titanate nanotubes for high efficient and high selective removal of U(VI). Chem Eng J 322:353–365
Tong J, Yang JQ, Zhang LL, Liu TH, Peng CY, Ni XF, Dong TH, Mocilac P, Shi KL, Hou XL (2022) Efficient removal of Se-79 from highly acidic solution using SiO2 particles functionalised with iron hydroxide. Chem Eng J 446:137387
Alharthi S (2021) Separation of thorium(IV) from aquatic media using magnetic ferrite nanoparticles. Radiochim Acta 109:823–833
Yang JQ, Shi KL, Wu F, Tong J, Su Y, Liu TH, He JG, Mocilac P, Hou XL, Wu WS, Shi WQ (2022) Technetium-99 decontamination from radioactive wastewater by modified bentonite: batch, column experiment and mechanism investigation. Chem Eng J 428:131333
Yen CH, Lien HL, Chung JS, Yeh HD (2017) Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J Hazard Mater 322:215–222
Li P, Wang JJ, Wang Y, Liang JJ, He BH, Pan DQ, Fan QH, Wang XK (2019) Photoconversion of U(VI) by TiO2: an efficient strategy for seawater uranium extraction. Chem Eng J 365:231–241
He NN, Li H, Cheng C, Dong H, Lu XR, Wen J, Wang XL (2020) Enhanced marine applicability of adsorbent for uranium via synergy of hyperbranched poly(amido amine) and amidoxime groups. Chem Eng J 395:125162
Wang Y, Lin ZW, Zhang HS, Liu Q, Yu J, Liu JY, Chen RR, Zhu JH, Wang J (2021) Anti-bacterial and super-hydrophilic bamboo charcoal with amidoxime modified for efficient and selective uranium extraction from seawater. J Colloid Interf Sci 598:455–463
Liu X, Wu J, Zhang S, Ding C, Sheng G, Alsaedi A, Hayat T, Li J, Song Y (2019) Amidoxime-functionalized hollow carbon spheres for efficient removal of uranium from wastewater. ACS Sustain Chem Eng 7:10800–10807
Acknowledgements
The financial support from National Natural Science Foundation of China (Nos. 22061132004, U21A20442, 22106059, 21771093), Science and Technology Projects of Gansu Province (21JR1RA265), Gansu Industrial Support Plan of colleges and Universities (2022CYZC-06) and Fundamental Research Funds for the Central Universities (Nos. lzujbky-2022-pd11, lzujbky-2022-kb13) are gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, F., Zhang, W., Zhou, Y. et al. Efficient removal of uranium from wastewater using amidoxime-carboxyl functional resin with large particle size. J Radioanal Nucl Chem 332, 1135–1147 (2023). https://doi.org/10.1007/s10967-022-08711-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08711-5