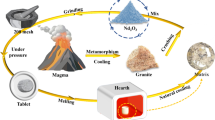
Abstract
Natural granite was considered for immobilizing nuclear waste Nd2O3 (Nd3+ simulated for An3+). The structure and performance of pure granite were compared with Nd2O3 doped granite sintered at 1500 °C. The introduced Nd3+ distributed mainly in the glass network below the loading capacity (19 wt.%). Beyond that, tetrahedral SiO4 units existed in a sheet form, while extra Nd3+ precipitated as Nd2Si2O7. The doped Nd2O3 improved the hardness (6.29–7.07 GPa) and density (2.19–2.71 g/cm3) of matrix. Moreover, the solidified form presented NRNd below 1.7 × 10–6 g m−2 d−1 after 28 days. This work disclosed a potential host matrix for nuclear waste.
Graphics abstract













Similar content being viewed by others
References
Liu ZX, Liu ZQ, Huang WH et al (2005) The key countermeasures of controlling fossil fuel environment pollution in China. Resour Ind 4:49–52
Temporal M, Canaud B, Ramis R (2020) Time evolution of the fuel areal density and electronic temperature provided by secondary nuclear fusion reaction. Eur Phys J D 73(10):1–10
Nuckols L, Crespillo ML, Xu C et al (2020) Coupled effects of electronic and nuclear energy deposition on damage accumulation in ion-irradiated SiC. Acta Mater 199:96–106
Budnitz RJ, Rogner HH, Shihab EA (2018) Expansion of nuclear power technology to new countries-SMRs, safety culture issues, and the need for an improved international safety regime. Energy Pol 119:535–544
Krauskopf KB (1990) Disposal of high-level nuclear waste: is it possible. Science 249:1231–1233
Ewing RC (2005) Plutonium and “minor” actinides: safe sequestration. Earth Planet Sci Lett 229(3):165–181
Murali MS, Bhattacharayya A, Raut DR et al (2012) Characterization of high level waste for minor actinides by chemical separation and alpha spectrometry. J Radioanal Nucl Ch 294(1):149–153
Laverov NP, Yudintsev SV, Livshits TS et al (2010) Synthetic minerals with the pyrochlore and garnet structures for immobilization of actinide-containing wastes. Geochem Int 48(1):1–14
El Afifi EM, Attallah MF, Borai EH et al (2016) Utilization of natural hematite as reactive barrier for immobilization of radionuclides from radioactive liquid waste. J Environ Radioactiv 151:156–165
Ewing RC (2001) The design and evaluation of nuclear-waste forms: clues from mineralogy. Can Mineral 39:697–715
Su S, Ding Y, Shu XY et al (2014) Nd and Ce simultaneous substitution driven structure modifications in Gd2-xNdxZr2-yCeyO7. J Eur Ceram Soc 35(6):1847–1853
Hench LL, Clark DE (1984) High level waste immobilization forms. Nucl Eng Technol 5(2):149–173
Yang D, Xia Y, Wen J et al (2017) Role of ion species in radiation effects of Lu2Ti2O7 pyrochlore. J Alloy Compd 693:565–572
Shu XY, Fan L, Hou CX et al (2017) Microstructure and performance studies of (Mo, Ru, Pd, Zr) tetra-doped gadolin-ium zirconate pyrochlore. Adv Appl Ceram 116:272–277
Trocellier P (2001) Chemical durability of high level nuclear waste forms. Annales de Chimie Science des Matériaux 26(2):113–130
Zhang YX, Liu SL, OuYang SL et al (2020) Transformation of unstable heavy metals in solid waste into stable state by the preparation of glass-ceramics-ScienceDirect. Mater Chem Phys 252:123061
Wang J, Chen L, Su R et al (2018) The Beishan underground research laboratory for geological disposal of high-level radioactive waste in China: planning, site selection, site characterization and in situ tests. J Rock Mech Geotech Eng 10(3):411–435
Luo X, Min M, Zheng Z et al (2004) Isotopic geochemistry of the deep granite under the pre-selected site of deep geological repositories for high-level radioactive waste. Acta Geol Sin 18(5):689–698
Wang W, Ding Z, Wang Y et al (2021) Transport behaviors of Cs+ in granite porous media: Effects of mineral composition, HA, and coexisting cations. Chemosphere 268:129341
Tsai SC, Wang TH, Wei YY et al (2008) Kinetics of Cs adsorption/desorption on granite by a pseudo first order reaction model. J Radioanal Nucl Ch 275(3):555–562
Lu XR, Chen SZ, Shu XY et al (2018) Immobilisation of nuclear waste by microwave sintering with a natural magmatic rock. Phil Mag Lett 98(4):155–160
Li LL, Shu XY, Cheng YR et al (2022) High immobilizing capacity of natural granite as glass-ceramic matrix to simulated trivalent actinide waste. Radiat Phys Chem 195:110067
Hestnes KH, Aasly K, Sandøy R et al (2013) Occurrence of iron in industrial granitic pegmatite. Miner Eng 52:21–30
Osmanlioglu AE (2006) Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey. J Hazard Mater 137:332–335
Zhang JF, Pang QB (2004) Zircon SHRIMP U-Pb dating of the granite porphyry in Baiyinbaolidao gold deposit, Inner Mongolia: the age of the ore-forming host rocks. Geol Bull China 23(2):189–192
Moghadam MC, Tahmasbi Z, Ahmadi KA et al (2018) Petrogenesis of Rabor-Lalehzar magmatic rocks (SE Iran): constraints from whole rock chemistry and Sr-Nd isotopes. Chemie der Erde-Geochem 78(1):58–77
Chen B, Xu B (1996) Basic characteristics and tectonic significance of two types of Paleozoic granitoids in Suzuo Qi area, Inner Mongolia. Acta Petrol Sin 12(4):16
Shi G, Miao L, Zhang F et al (2004) Emplacement age and tectonic implications of the Xilinhot A-type granite in Inner Mongolia, China. Sci Bull 49(7):7
Gion AM, Piccoli PM, Candela PA (2022) Characterization of biotite and amphibole compositions in granites. Contrib Mineral Petr. https://doi.org/10.1007/s00410-022-01908-7
Lu XR, Cui CL, Zhang D et al (2010) Structural evolution of radioactive zirconite in geological environment and its ability to resist γ-ray irradiation. J Southwest Univ Sci Technol 25(3):33–38
Qasrawi AF, Kmail BH, Mergen A (2012) Synthesis and characterization of Bi1.5Zn0.92Nb1.5-xSnxO6.92-x/2 pyrochlore ceramics. Ceram Int 38:4181–4187
Jain S, Jha AK (2008) Structural and electrical properties of SrBi2VxNb2-xO9 ferroelectric ceramics: effect of temperature and frequency. J Electroceram 24(1):58–63
Kerr RA (2000) Science and policy clash at Yucca mountain. Science 288:602
ASTM C1285–2002, Standard test methods for determining chemical durability of nuclear, hazardous, and mixed waste glasses and multiphase glass ceramics: the product consistency test (PCT) [S]. 2008
Pan X, Cui W, Zhang C et al (2020) Formation kinetics and transition mechanism of CaO·SiO2 in low-calcium system during high-temperature sintering. J Cent South Univ 27(11):3269–3277
Dias JDM, Melo GHA, Lodi TA et al (2016) Thermal and structural properties of Nd2O3-doped calcium boroaluminate glasses. J Rare Earth 34(5):521–528
Felsche J, Hirsiger W (1969) The polymorphs of the rare-earth pyrosilicates R.E.2Si2O7, [R.E.: La, Ce, Pr, Nd, Sm]. J Less-Common Met 18:131
Ke SJ, Wang YM, Pan ZD et al (2017) Effect of mechanical activation on solid-state synthesis process of neodymium disilicate ceramic pigment. Dyes Pigments 145:160–167
He Y, Shu X, Li LL et al (2022) Doping effect of neodymium ions as simulated an3+ on the structure and performance of the granite solidified substrate. SSRN Electr J. https://doi.org/10.2139/ssrn.4045428
Kaur P, Kaur S, Singh GP et al (2014) Cerium and samarium codoped lithium aluminoborate glasses for white light emitting devices. J Alloys Compd 588:394–398
Liang LY, Liu ZM, Ca HT et al (2010) Microstructural, optical, and electrical properties of SnO thin films prepared on quartz via a two-step method. Acs Appl Mater Inter 2(4):1060
Sharaf El Deen LM, Al Salhi MS, Meawad M et al (2008) IR and UV spectral studies for rare earths-doped tellurite glasses. J Alloys Compd 465:333–339
Vicente Rodríguez MA, Suarez M, Bañares Muñoz MA et al (1996) Comparative FT-IR study of the removal of octahedral cations and structural modifications during acid treatment of several silicates. Spectrochim Acta A 52(13):1685–1694
Chen H, Sun Z, Shao J et al (2011) Investigation on FT-IR spectroscopy for eight different sources of SiO2. Bull Chin Ceram Soc 30(4):934–937
Etchepare J (1970) Interprétation des spectres de diffusion Raman de verres de silice binaires. Spectrochim Acta Part A Mol Spectrosc 26(11):2147–2154
Mcmillan P (1984) Structural studies of silicate glasses and melts-Applications and limitations of Raman spectroscopy. Am Mineral 69(69):622–644
Nakamoto K (2008) Infrared and Raman spectra of inorganic and coordination compounds. Theory Appl Inorg Chem 5:88–97
Wang WT, Zhang H, Yuan Y et al (2018) Research progress of Raman spectroscopy in drug analysis. AAPS PharmSciTech 19:2921
Denry IL, Holloway JA (2004) Elastic constants, Vickers hardness, and fracture toughness of fluorrichterite-based glass-ceramics. Dent Mater 20(3):213–219
Le P, Zhang K, He Z et al (2017) Self-propagating high-temperature synthesis of ZrO2 incorporated Gd2Ti2O7 pyrochlore. J Adv Ceram 7(1):41–49
Yamane M, Mackenzie JD (1974) Vicker’s hardness of glass. J Non-Cryst Solids 15(2):153–164
Lian QH, Zhang XQ, Ji HJ (2020) Effect of V2O5 on crystallization tendency and chemical durability of Mo-bearing aluminoborosilicate glass. Mater Res Express 7:1–9
Shaaban KS, Yousef ES (2020) Optical properties of Bi2O3 doped boro tellurite glasses and glass ceramics. Optik 203:163976
Neuville DR, Cormier L, Massiot D (2004) Al environment in tectosilicate and peraluminous glasses: A 27Al MQ-MAS NMR, Raman, and XANES investigation. Geochim Cosmochim Acta 68(24):5071–5079
Li B, Wang Z, Xia Q (2019) Influence of Nd2O3 addition on sintering kinetics, microstructures, and properties of CaO-B2O3-SiO2 glass-ceramics for packages. J Electron Mater 48(12):7923–7928
Scannell G, Laille D, Célarié F et al (2017) Interaction between deformation and crack initiation under Vickers indentation in Na2O-TiO2-SiO2 glasses. Front Mater 4:6
Tiegel M, Hosseinabadi R, Kuhn S et al (2015) Young׳s modulus, Vickers hardness and indentation fracture toughness of alumino silicate glasses. Ceram Int 41(6):7267–7275
Ojovan MI, Lee WE (2011) Glassy waste forms for nuclear waste immobilization. Metall Mater Trans A 42:837–851
Acknowledgements
The authors appreciate the supports from the National Natural Science Foundation of China (No. 21976146), the Project of State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology (No. 21fksy13) and the State Environmental Protection Key Laboratory of Synergetic Control and Joint Remediation for Soil & Water Pollution, Chengdu University of Technology (CHBK-2020-005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Shu, X., Li, L. et al. Nd2O3 immobilized by granite based glass–ceramics: composition, structure, and performance. J Radioanal Nucl Chem 332, 105–117 (2023). https://doi.org/10.1007/s10967-022-08657-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08657-8