Abstract
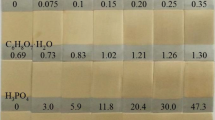
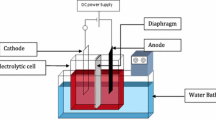
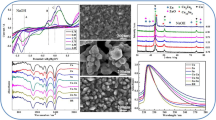
The article proposed a new electrodeposition solution based on a citric–oxalic–sulfate matrix intended for high-resolution electroplating of actinides. The suggested method originated from the well-established oxalate-ammonium sulfate solution, but instead of DTPA and hydroxyl ammonium sulfate, citric acid was used. Since the volume of electrolyte, distance, and current density were discovered to be the same as for standard oxalate-ammonium sulfate solution, the focus was to evaluate the optimal concentration of all compounds, the working range of pH, the chemical yield, resolution, and the uniformity of deposited layer of the selected actinides, namely Am, Pu, U, and Cm. The obtained results were better than using the standard plating solution while eliminating expensive and harmful chemicals. The chemical yield of this method was observed to be quantitative for all selected radionuclides.



Similar content being viewed by others
References
Wilson RE, Schwindt O, Fenter P, Soderholm L (2010) Exploitation of the sorptive properties of mica for the preparation of higher-resolution alpha-spectroscopy samples. Radiochim Acta, 98. https://doi.org/10.1524/ract.2010.1736
Nilsson H, Ramebäck H, Skålberg M (2001) An improved method for α-source preparation using neodymium fluoride coprecipitation. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip, 462. https://doi.org/10.1016/S0168-9002(01)00184-X
Lee MH, Park TH, Song BC et al (2012) A study of simple a source preparation using a micro-coprecipitation method. Bull Korean Chem Soc, 33. https://doi.org/10.5012/bkcs.2012.33.11.3745
Hansen PG (1959) The conditions for electrodeposition of insoluble hydroxides at a cathode surface: A theoretical investigation. J Inorg Nucl Chem, 12. https://doi.org/10.1016/0022-1902(59)80089-0
Crespo MT (2012) A review of electrodeposition methods for the preparation of alpha-radiation sources. Appl Radiat Isot, 70. https://doi.org/10.1016/j.apradiso.2011.09.010
Ko YG (2020) Preparation and characterization of electrodeposited layers as alpha sources for alpha-particle spectrometry. J Radioanal Nucl Chem, 326. https://doi.org/10.1007/s10967-020-07398-w
Dumitru OA, Begy RC, Nita DC et al (2013) Uranium electrodeposition for alpha spectrometric source preparation. J Radioanal Nucl Chem, 298. https://doi.org/10.1007/s10967-013-2584-x
Janda J, Sládek P, Sas D (2010) Electrodeposition of selected alpha-emitting radionuclides from oxalate-ammonium sulfate electrolyte and measured by means of solid-state alpha spectrometry. J Radioanal Nucl Chem, 286. https://doi.org/10.1007/s10967-010-0756-5
Payne RF, Lamont SP, Filby RH, Glover SE (2001) Optimization and characterization of a sulfate based electrodeposition method for alpha-spectrometry of neptunium and curium. Radioanal Nucl Chem, 248. https://doi.org/10.1023/A:1010608931914
Oh JS, Warwick PE, Croudace IW, Lee SH (2014) Evaluation of three electrodeposition procedures for uranium, plutonium and americium. Appl Radiat Isot, 87. https://doi.org/10.1016/j.apradiso.2013.11.048
Kressln IK (1977) Electrodeposition of Plutonium and Americium for High Resolution α Spectrometry. Anal Chem, 49. https://doi.org/10.1021/ac50014a044
Lee MH, Lee CW (2000) Preparation of alpha-emitting nuclides by electrodeposition. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip, 447. https://doi.org/10.1016/S0168-9002(99)01190-0
Lee MH, Pimpl M (1999) Development of a new electrodeposition method for Pu-determination in environmental samples. Appl Radiat Isot, 50. https://doi.org/10.1016/S0969-8043(98)00155-9
Mirashi NN, Aggarwal SK (2009) Studies for simultaneous quantitative electrodeposition of plutonium and americium for alpha-spectrometry. J Radioanal Nucl Chem, 279. https://doi.org/10.1007/s10967-008-7338-9
Salar Amoli H, Barker J, Flowers A (2006) Electrodeposition and determination of nano-scale uranium and plutonium using alpha-spectroscopy. J Radioanal Nucl Chem, 268. https://doi.org/10.1007/s10967-006-0197-3
Trdin M, Benedik L, Samardžija Z, Pihlar B (2012) Investigation of factors affecting the quality of americium electroplating. Appl Radiat Isot, 70. https://doi.org/10.1016/j.apradiso.2012.02
Saliba-Silva AM, de Oliveira ET, Durazzo M (2014) Uranium Electrodeposition Using Direct Potential Technique in Less Acidic Aqueous Media. ECS Trans, 61. https://doi.org/10.1149/06124.0001ecst
Lee MH, Kim CJ, Boo BH (2000) Electrodeposition of alpha-emitting nuclides from ammonium oxalate-ammonium sulfate electrolyte. Bull Korean Chem Soc 21:175–179
Maya L, Gonzalez BD, Lance MJ, Holcomb DE (2004) Electrodeposition of uranium dioxide films. J Radioanal Nucl Chem, 261. https://doi.org/10.1023/B:JRNC.0000037102.88633.2b
Vera Tome F, Martin Sanchez A (1991) Optimizing the parameters affecting the yield and energy resolution in the electrodeposition of uranium. Int J Radiat Appl Instrumentation Part, 42. https://doi.org/10.1016/0883-2889(91)90062-6
Da Cruz PAL, Poledna R (1990) Alpha-source preparation by electrodeposition. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers Detect Assoc Equip 286:453–456
Talvitie NA (1972) Electrodeposition of Actinides for Alpha Spectrometric Determination. Anal Chem, 44. https://doi.org/10.1021/ac60310a013
Zhu S, Yue J, Qin X et al (2016) The Role of Citric Acid in Perfecting Platinum Monolayer on Palladium Nanoparticles during the Surface Limited Redox Replacement Reaction. J Electrochem Soc, 163. https://doi.org/10.1149/2.0061612jes
Du J, Xu L, Chen B, Li D (2013) Electrodeposition of nanocrystalline Ni-W coatings with citric acid system. J Nanosci Nanotechnol, 13. https://doi.org/10.1166/jnn.2013.6850
Müller T, Grimwood J, Bachmaier A, Pippan R (2018) Electrodeposition of Fe-C alloys from citrate baths: Structure, mechanical properties, and thermal stability. Metals (Basel), 8. https://doi.org/10.3390/met8050363
Rulfs CL, De AK, Elving PJ (1957) Electrodeposition of uranium at the microgram level. J Electrochem Soc, 104. https://doi.org/10.1149/1.2428517
Janda J (2021) Evaluation of sulfate-oxalate deposition solution with new electrodeposition system. J Radioanal Nucl Chem, 329. https://doi.org/10.1007/s10967-021-07777-x0
Lally AE, Glover KM (1984) Source preparation in alpha spectrometry. Nucl Instruments Methods Phys Res, 223. https://doi.org/10.1016/0167-5087(84)90658-6
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janda, J. The use of a citric–oxalic–sulfate as a new electrodeposition solution. J Radioanal Nucl Chem 332, 1427–1433 (2023). https://doi.org/10.1007/s10967-022-08644-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08644-z