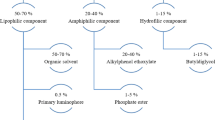
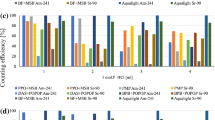
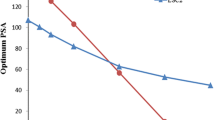
Abstract
The paper describes the influence of secondary organic solvents on the performance of a newly designed scintillation cocktail. The work mainly focuses on the sample load capacity of the scintillation cocktail in connection with the solvent component to be able to accept the largest possible volume of different types of samples, especially aqueous ones. Previous research was focused on the ideal composition of the detergent component, which has a major impact on sample load capacity and new primary phosphors for use in liquid scintillation. However, the solvent component also has a significant impact on sample load capacity. Individual properties characterizing the performance of the scintillation cocktail were tested namely detection efficiency, alpha/beta discrimination ability, spillover effect, FOM, and sample load capacity. The resulting scintillation cocktail was compared to selected commercially available scintillation cocktails.






Similar content being viewed by others
References
Kallmann H(1950) Scintillation counting with solutions [11].Phys. Rev.78
Reynolds GT, Harrison FB, Salvini G(1950) Liquid scintillation counters [28].Phys. Rev.78
Broda R, Cassette P, Kossert K (2007) Radionuclide metrology using liquid scintillation counting. Metrologia 44. Doi: https://doi.org/10.1088/0026-1394/44/4/S06
Hou X(2018) Liquid scintillation counting for determination of radionuclides in environmental and nuclear application.J. Radioanal. Nucl. Chem.318
L’Annunziata MF, Tarancón A, Bagán H, García JF (2020) Liquid scintillation analysis: principles and practice. Handbook of Radioactivity Analysis, pp. 575–801. Academic press. Doi: https://doi.org/10.1016/b978-0-12-814397-1.00006-6
Gibson JAB (1971) Liquid Scintillation Counting as an Absolute Method. Liquid scintillation counting vol, vol 2. Heyden and Son, London, UK, pp 23–37
Lombardi P, Ranucci G, Ortica F, Romani A (2013) Decay time and pulse shape discrimination of liquid scintillators based on novel solvents. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip 701. Doi:https://doi.org/10.1016/j.nima.2012.10.061
Thomson J, Temple S (2020) Sample preparation techniques for liquid scintillation analysis. In: Handbook of Radioactivity Analysis: Volume 1: Radiation Physics and Detectors, pp. 803–860. Doi:https://doi.org/10.1016/b978-0-12-814397-1.00007-8
Kaptanoglu T, Callaghan EJ, Yeh M, Orebi Gann GD (2022) Cherenkov and scintillation separation in water-based liquid scintillator using an LAPPDTM. Eur Phys J C 82. doi: https://doi.org/10.1140/epjc/s10052-022-10087-5
Boos EF, Magid J, Bruun S, Jørgensen NOG (2022) Liquid scintillation counting can underestimate 14 C-activity of 14CO2 trapped in NaOH. Soil Biol Biochem 166. doi: https://doi.org/10.1016/j.soilbio.2022.108576
Horrocks D (ed) (2012) Applications of liquid scintillation counting. Elsevier
Bush ET (1963) General Applicability of the Channels Ratio Method of Measuring Liquid Scintillation Counting Efficiencies. Anal Chem 35(8):1024–1029. Doi: https://doi.org/10.1021/ac60201a032
Cook GT, Anderson R (1991) The determination of 241Pu by liquid scintillation spectrometry using the Packard 2250CA. J Radioanal Nucl Chem Lett 154(5):319–330. Doi: https://doi.org/10.1007/bf02165488
Verrezen F, Loots H, Hurtgen C (2008) A performance comparison of nine selected liquid scintillation cocktails. Appl Radiat Isot 66. doi: https://doi.org/10.1016/j.apradiso.2008.02.050
Wiel JT, Hegge T (1991) Advances in scintillation cocktails. Liquid scintillation counting and organic scintillators, first edn. Lewis, Michigan, pp 51–67
Aqualight HIDEX, Aqualight+ AB (2021) MaxiLight + Cocktails for LSC. https://hidex.com/wp-content/uploads/2019/10/HIDEX-Cocktails-1.pdf. Accessed 22
Perkin Elmer - MSDS Ultima Gold XR (2021) https://www.perkinelmer.com/Content/MSDSDatabase/MSDS_6013119_Ultima_Gold_XR_(GB).pdf. Accessed 22
Rajchl E, Janda J, Sedlačík M (2021) Optimization of composition of water accepting scintillation cocktail. J Radioanalytical Nuclear Chem Doi:. doi.https://doi.org/10.1007/s10967-022-08386-y
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajchl, E., Janda, J. Effect of secondary solvent on the sample load capacity of a newly designed scintillation cocktail. J Radioanal Nucl Chem 332, 1445–1451 (2023). https://doi.org/10.1007/s10967-022-08563-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08563-z