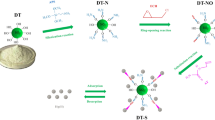
Abstract
In this work, an inexpensive PEI-AC sorbent was synthesized by facile hydrothermal grafting method and used to uranium capture from seawater. Batch experimental demonstrated that the optimal operation pH of PEI-AC was 6.0. The adsorption process is conformed to pseudo-second-order kinetic model and Langmuir monolayer adsorption isotherm, and the maximum equilibrium adsorption capacity reached 50.27 mg/g. The PEI-AC samples exhibited better selective and efficiency in various competing ions and uranium-spiked seawater. Furthermore, the removal efficiency remained 88.52% after five sequence adsorption/desorption experiments. By the analysis of XPS, the interaction of UO22+ with N/O-containing ligands were the primary immobilization mechanism.







Similar content being viewed by others
References
Cui WR, Li FF, Xu RH, Zhang CR, Chen XR, Yan RH, Liang RP, Qiu JD (2020) Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew Chem Int Edit 59:17684–17690. https://doi.org/10.1002/anie.202007895
Tan L, Liu Q, Jing X, Liu J, Song D, Hu S, Liu L, Wang J (2015) Removal of uranium(VI) ions from aqueous solution by magnetic cobalt ferrite/multiwalled carbon nanotubes composites. Chem Eng J 273:307–315. https://doi.org/10.1016/j.cej.2015.01.110
Wang XX, Chen L, Wang L, Fan QH, Pan DQ, Li JX, Chi FT, Xie Y, Yu SJ, Xiao CL, Luo F, Wang J, Wang XL, Chen CL, Wu WS, Shi WQ, Wang S, Wang XK (2019) Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci China Chem 62:933–967. https://doi.org/10.1007/s11426-019-9492-4
Yuan YH, Liu TT, Xiao JX, Yu QH, Feng LJ, Niu BY, Feng SW, Zhang JC, Wang N (2020) DNA nano-pocket for ultra-selective uranyl extraction from seawater. Nat Commun. https://doi.org/10.1038/s41467-020-19419-z
Zhao SL, Yuan YH, Yu QH, Niu BY, Liao JH, Guo ZH, Wang N (2019) A dual-surface amidoximated halloysite nanotube for high-efficiency economical uranium extraction from seawater. Angew Chem Int Edit 58:14979–14985. https://doi.org/10.1002/anie.201908762
Yuan YH, Yu QH, Yang S, Wen J, Guo ZH, Wang XL, Wang N (2019) Ultrafast recovery of uranium from seawater by bacillus velezensis strain UUS-1 with innate anti-biofouling activity. Adv Sci. https://doi.org/10.1002/advs.201900961
Chen F, Lv M, Ye Y, Miao S, Tang X, Liu Y, Liang B, Qin Z, Chen Y, He Z, Wang Y (2022) Insights on uranium removal by ion exchange columns: The deactivation mechanisms, and an overlooked biological pathway. Chem Eng J 434:134708. https://doi.org/10.1016/j.cej.2022.134708
Zarrougui R, Mdimagh R, Raouafi N (2018) Highly efficient extraction and selective separation of uranium (VI) from transition metals using new class of undiluted ionic liquids based on H-phosphonate anions. J Hazard Mater 342:464–476. https://doi.org/10.1016/j.jhazmat.2017.08.057
Shi S, Qian Y, Mei P, Yuan Y, Jia N, Dong M, Fan J, Guo Z, Wang N (2020) Robust flexible poly(amidoxime) porous network membranes for highly efficient uranium extraction from seawater. Nano Energy 71:104629. https://doi.org/10.1016/j.nanoen.2020.104629
Liang P-l, Yuan L-y, Deng H, Wang X-c, Wang L, Li Z-j, Luo S-z, Shi W-q (2020) Photocatalytic reduction of uranium(VI) by magnetic ZnFe2O4 under visible light. Appl Catal B-Environ 267:118688. https://doi.org/10.1016/j.apcatb.2020.118688
Meng Q, Yang X, Wu L, Chen T, Li Y, He R, Zhu W, Zhu L, Duan T (2022) Metal-free 2D/2D C3N5/GO nanosheets with customized energy-level structure for radioactive nuclear wastewater treatment. J Hazard Mater 422:126912. https://doi.org/10.1016/j.jhazmat.2021.126912
Wu L, Yang X, Chen T, Li Y, Meng Q, Zhu L, Zhu W, He R, Duan T (2022) Three-dimensional C3N5/RGO aerogels with enhanced visible-light response and electron-hole separation efficiency for photocatalytic uranium reduction. Chem Eng J. https://doi.org/10.1016/j.cej.2021.131773
He P, Zhang L, Wu L, Yang X, Chen T, Li Y, Yang X, Zhu L, Meng Q, Duan T (2022) Synergistic Effect of the Sulfur Vacancy and Schottky Heterojunction on Photocatalytic Uranium Immobilization: The Thermodynamics and Kinetics. Inorg Chem 61:2242–2250. https://doi.org/10.1021/acs.inorgchem.1c03552
Ye Y, Fan B, Qin Z, Tang X, Feng Y, Lv M, Miao S, Li H, Chen Y, Chen F, Wang Y (2022) Electrochemical removal and recovery of uranium: Effects of operation conditions, mechanisms, and implications. J Hazard Mater 432:128723. https://doi.org/10.1016/j.jhazmat.2022.128723
Hamza MF, Roux J-C, Guibal E (2018) Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem Eng J 344:124–137. https://doi.org/10.1016/j.cej.2018.03.029
Abney CW, Mayes RT, Saito T, Dai S (2017) Materials for the Recovery of Uranium from Seawater. Chem Rev 117:13935–14013. https://doi.org/10.1021/acs.chemrev.7b00355
Tsarev S, Waite TD, Collins RN (2016) Uranium Reduction by Fe(II) in the Presence of Montmorillonite and Nontronite. Environ Sci Technol 50:8223–8230. https://doi.org/10.1021/acs.est.6b02000
Tran EL, Teutsch N, Klein-BenDavid O, Weisbrod N (2018) Uranium and Cesium sorption to bentonite colloids under carbonate-rich environments: Implications for radionuclide transport. Sci Total Environ 643:260–269. https://doi.org/10.1016/j.scitotenv.2018.06.162
Crane RA, Pullin H, Scott TB (2015) The influence of calcium, sodium and bicarbonate on the uptake of uranium onto nanoscale zero-valent iron particles. Chem Eng J 277:252–259. https://doi.org/10.1016/j.cej.2015.03.085
Kumar V, Singh V, Kim K-H, Kwon EE, Younis SA (2021) Metal-organic frameworks for photocatalytic detoxification of chromium and uranium in water. Coordin Chem Rev 447:214148. https://doi.org/10.1016/j.ccr.2021.214148
Guo H, Mei P, Xiao J, Huang X, Ishag A, Sun Y (2021) Carbon materials for extraction of uranium from seawater. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.130411
Dai L, Li L, Zhu W, Ma H, Huang H, Lu Q, Yang M, Ran Y (2020) Post-engineering of biochar via thermal air treatment for highly efficient promotion of uranium(VI) adsorption. Bioresource Technol 298:122576. https://doi.org/10.1016/j.biortech.2019.122576
Zhu M, Li F, Chen W, Yin X, Yi Z, Zhang S (2021) Adsorption of U(VI) from aqueous solution by using KMnO4-modified hazelnut shell activated carbon: characterisation and artificial neural network modelling. Environ Sci Pollut R 28:47354–47366. https://doi.org/10.1007/s11356-021-14034-x
Chu G, Zhao J, Huang Y, Zhou D, Liu Y, Wu M, Peng H, Zhao Q, Pan B, Steinberg CEW (2018) Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ Pollut 240:1–9. https://doi.org/10.1016/j.envpol.2018.04.003
Jin J, Li S, Peng X, Liu W, Zhang C, Yang Y, Han L, Du Z, Sun K, Wang X (2018) HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresource Technol 256:247–253. https://doi.org/10.1016/j.biortech.2018.02.022
Yu S, Wu X, Ye J, Li M, Zhang Q, Zhang X, Lv C, Xie W, Shi K, Liu Y (2022) Dual effect of acetic acid efficiently enhances sludge-based biochar to recover uranium from aqueous solution. Front Chem. https://doi.org/10.3389/fchem.2022.835959
Alahabadi A, Singh P, Raizada P, Anastopoulos I, Sivamani S, Dotto GL, Landarani M, Ivanets A, Kyzas GZ, Hosseini-Bandegharaei A (2020) Activated carbon from wood wastes for the removal of uranium and thorium ions through modification with mineral acid. Colloids Surf a-Physicochem Eng Aspects. https://doi.org/10.1016/j.colsurfa.2020.125516
Nezhad MM, Semnani A, Tavakkoli N, Shirani M (2021) Selective and highly efficient removal of uranium from radioactive effluents by activated carbon functionalized with 2-aminobenzoic acid as a new sorbent. J Environ Manage 299:113587–113587. https://doi.org/10.1016/j.jenvman.2021.113587
Liatsou I, Pashalidis I, Dosche C (2020) Cu(II) adsorption on 2-thiouracil-modified Luffa cylindrica biochar fibres from artificial and real samples, and competition reactions with U(VI). J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.120950
Abu-Dalo MA, Nevostrueva S, Hernandez M (2020) Removal of radionuclides from acidic solution by activated carbon impregnated with methyl- and carboxy-benzotriazoles. Sci Rep-UK. https://doi.org/10.1038/s41598-020-68645-4
He N, Li H, Cheng C, Dong H, Lu X, Wen J, Wang X (2020) Enhanced marine applicability of adsorbent for uranium via synergy of hyperbranched poly(amido amine) and amidoxime groups. Chem Eng J 395:125162. https://doi.org/10.1016/j.cej.2020.125162
Li H, Li Y, Zhou Y, Li B, Liu D, Liao H (2019) Efficient removal of uranium using a melamine/trimesic acid-modified hydrothermal carbon-based supramolecular organic framework. J Colloid Interf Sci 544:14–24. https://doi.org/10.1016/j.jcis.2019.02.079
Zhou Y, Li Y, Liu D, Liu D, Xu L, Liu C (2021) Adsorption optimization of uranium(VI) onto polydopamine and sodium titanate co-functionalized MWCNTs using response surface methodology and a modeling approach. Colloid Surface A 627:127145. https://doi.org/10.1016/j.colsurfa.2021.127145
Bayramoglu G, Arica MY (2017) Polyethylenimine and tris(2-aminoethyl)amine modified p(GA–EGMA) microbeads for sorption of uranium ions: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 312:293–303. https://doi.org/10.1007/s10967-017-5216-z
Sun G, Zhou L, Tang X, Le Z, Liu Z, Huang G (2020) In situ formed magnetic chitosan nanoparticles functionalized with polyethylenimine for effective U(VI) sorption. J Radioanal Nucl Chem 325:595–604. https://doi.org/10.1007/s10967-020-07230-5
Pang H, Huang S, Wu Y, Yang D, Wang X, Yu S, Chen Z, Alsaedi A, Hayat T, Wang X (2018) Efficient elimination of U(vi) by polyethyleneimine-decorated fly ash. Inorg Chem Front 5:2399–2407. https://doi.org/10.1039/C8QI00253C
Sun Y, Zhang H, Yuan N, Ge Y, Dai Y, Yang Z, Lu L (2021) Phosphorylated biomass-derived porous carbon material for efficient removal of U(VI) in wastewater. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.125282
Zhang Q, Wang Y, Wang Z, Zhang Z, Wang X, Yang Z (2021) Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. J Alloy Compd 852:156993. https://doi.org/10.1016/j.jallcom.2020.156993
Su S, Liu Q, Liu J, Zhang H, Li R, Jing X, Wang J (2018) Polyethyleneimine-functionalized Luffa cylindrica for efficient uranium extraction. J Colloid Interf Sci 530:538–546. https://doi.org/10.1016/j.jcis.2018.03.102
Kong Y, Jin L, Qiu J (2013) Synthesis, characterization, and CO2 capture study of micro-nano carbonaceous composites. Sci Total Environ 463–464:192–198. https://doi.org/10.1016/j.scitotenv.2013.05.050
Tan X, Fan Q, Wang X, Grambow B (2009) Eu(III) Sorption to TiO2 (Anatase and Rutile): Batch, XPS, and EXAFS Studies. Environ Sci Technol 43:3115–3121. https://doi.org/10.1021/es803431c
Yan T, Luo X, Zou Z, Lin X, He Y (2017) Adsorption of Uranium(VI) from a Simulated Saline Solution by Alkali-Activated Leather Waste. Ind Eng Chem Res 56:3251–3258. https://doi.org/10.1021/acs.iecr.6b04425
Venkata Sravani V, Sengupta S, Sreenivasulu B, Gopakumar G, Tripathi S, Chandra M, Brahmmananda Rao CVS, Suresh A, Nagarajan S (2022) Highly efficient functionalized MOF-LIC-1 for extraction of U(vi) and Th(iv) from aqueous solution: experimental and theoretical studies. Dalton T 51:3557–3571. https://doi.org/10.1039/D1DT03317D
Shao D, Jiang Z, Wang X, Li J, Meng Y (2009) Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO22+ from aqueous solution. J Phys Chem B 113:860–864. https://doi.org/10.1021/jp8091094
Ma L, Huang Y, Zhao K, Deng H, Tian Q, Yan M (2021) Removal of uranium from acidic aqueous solution by natural fluorapatite. J Environ Chem Eng 9:106600. https://doi.org/10.1016/j.jece.2021.106600
Al-Harahsheh M, AlJarrah M, Alrebaki M, Mayyas M (2020) Nanoionic exchanger with unprecedented loading capacity of uranium. Sep Purif Technol 238:116423. https://doi.org/10.1016/j.seppur.2019.116423
Xue J-H, Zhang H, Ding DX, Hu N, Wang Y-D, Wang Y-S (2019) Linear β-cyclodextrin polymer functionalized multiwalled carbon nanotubes as nanoadsorbent for highly effective removal of U(VI) from aqueous solution based on inner-sphere surface complexation. Ind Eng Chem Res 58:4074–4083. https://doi.org/10.1021/acs.iecr.8b05453
Chen H, Chen Z, Zhao G, Zhang Z, Xu C, Liu Y, Chen J, Zhuang L, Haya T, Wang X (2018) Enhanced adsorption of U(VI) and 241Am(III) from wastewater using Ca/Al layered double hydroxide@carbon nanotube composites. J Hazard Mater 347:67–77. https://doi.org/10.1016/j.jhazmat.2017.12.062
Wang X, Liu Q, Liu J, Chen R, Zhang H, Li R, Li Z, Wang J (2017) 3D self-assembly polyethyleneimine modified graphene oxide hydrogel for the extraction of uranium from aqueous solution. Appl Surf Sci 426:1063–1074. https://doi.org/10.1016/j.apsusc.2017.07.203
Feng S, Feng L, Wang M, Yuan Y, Yu Q, Feng T, Cao M, Wang N, Peng Q (2022) Highly efficient extraction of uranium from seawater by natural marine crab carapace. Chem Eng J 430:133038. https://doi.org/10.1016/j.cej.2021.133038
Amphlett JTM, Choi S, Parry SA, Moon EM, Sharrad CA, Ogden MD (2020) Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: Chelation vs. anion exchange. Chem Eng J 392:123712. https://doi.org/10.1016/j.cej.2019.123712
Tangtubtim S, Saikrasun S (2019) Adsorption behavior of polyethyleneimine-carbamate linked pineapple leaf fiber for Cr(VI) removal. Appl Surf Sci 467–468:596–607. https://doi.org/10.1016/j.apsusc.2018.10.204
Wang S, Xiao K, Mo Y, Yang B, Vincent T, Faur C, Guibal E (2020) Selenium(VI) and copper(II) adsorption using polyethyleneimine-based resins: Effect of glutaraldehyde crosslinking and storage condition. J Hazard Mater 386:121637. https://doi.org/10.1016/j.jhazmat.2019.121637
Xie X, Gao H, Luo X, Su T, Zhang Y, Qin Z (2019) Polyethyleneimine modified activated carbon for adsorption of Cd(II) in aqueous solution. J Environ Chem Eng 7:103183. https://doi.org/10.1016/j.jece.2019.103183
Manos MJ, Kanatzidis MG (2012) Layered Metal Sulfides Capture Uranium from Seawater. J Am Chem Soc 134:16441–16446. https://doi.org/10.1021/ja308028n
Ahmad M, Ren J, Zhang Y, Kou H, M-u-d N, Zhang Q, Zhang B (2022) Simple and facile preparation of tunable chitosan tubular nanocomposite microspheres for fast uranium(VI) removal from seawater. Chem Eng J 427:130934. https://doi.org/10.1016/j.cej.2021.130934
Marwa MA (2021)Adsorption phenomena: definition, mechanisms, and adsorption types: short review. Green Appl Chem 13:43–51.ISSN: 2605–6895
Acknowledgements
This work was funded by the Ministry of Science and Technology of the People’s Republic of China (Grant No. 2016YFC1402504), and we highly appreciated the assist of characterization by Key Lab of material composite new technology of Wuhan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors of this article declared that there has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Li, Y., Liu, S. et al. Efficient extraction of UO22+ from seawater by polyethylenimine functionalized activated carbon (PEI-AC): adsorption performance and mechanism. J Radioanal Nucl Chem 331, 4635–4648 (2022). https://doi.org/10.1007/s10967-022-08523-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08523-7