Abstract
In this paper, activated carbon has been modified by water immersion and freeze–thaw cycles to further improve its radon adsorption capacity. Under freezing at − 80 °C and thawing at 50 °C, three cycles of modification were most effective, the radon adsorption coefficient was increased by 24%, and remained stable after five repeated radon adsorption–desorption cycles. The apparent morphology of the modified activated carbon changed significantly. The specific surface area of micropores increased significantly and was positively correlated with the radon adsorption capacity. The new application of this method can provide some references for the modification of material.
Graphical abstract
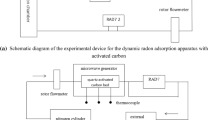
Experimental flow process diagram. The activated carbon was modified by water immersion and freeze–thaw cycles under different experimental conditions, and the best modification process was determined by measuring the static adsorption coefficient. The activated carbon with better modification effect was characterized and tested to observe the morphological and structural changes. It was finally concluded that water immersion and freeze-thawing could effectively enhance the microporous volume, specific surface area and radon adsorption capacity of activated carbon.








Similar content being viewed by others
References
Ting DSK (2010) WHO handbook on indoor radon: a public health perspective. Int J Environ Stud 67(1):100–102
Műllerová M, Mazur J, Blahušiak P, Grządziel D, Holý K, Kovács T, Kozak K, Csordás A, Neznal M, Neznal M, Shahrokhi A (2016) Indoor radon activity concentration in thermal spas: the comparison of three types of passive radon detectors. J Radioanal Nucl Chem 310(3):1077–1084
Kim KH, Pyeon SJ, Kim YH, Lee SS (2019) Radon reduction performance of adsorbent for making radon-reducing functional board. J Korea Inst Build Constr 19(2):139–147
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619
Wang Q, Qu J, Zhu W, Zhou B, Cheng J (2011) An experimental study on radon adsorption ability and microstructure of activated carbon. Nucl Sci Eng 168(3):287–292
Qiao B, Liu C, Zheng W, Li Q, Lin P, Wang Z, Chen J, Zhou D, Liu X, Li Y (2021) Dynamic adsorption properties of activated carbon for radioactive noble gas treatment in offshore floating nuclear power plant. J Radioanal Nucl Ch 327(1):207–215
Miranda HA (1957) The radon content of the atmosphere in the New York area as measured with an improved technique. J Atmos Terr Phys 11(3):272–283
Liu M, Xiao C (2018) Research progress on modification of activated carbon. In: E3S Web of Conferences. EDP Sciences, vol. 38, p. 02005
Heidari A, Younesi H, Rashidi A, Ghoreyshi AA (2014) Evaluation of CO2 adsorption with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. Chem Eng J 254:503–513
Ge X, Tian F, Wu Z, Yan Y, Cravotto G, Wu Z (2015) Adsorption of naphthalene from aqueous solution on coal-based activated carbon modified by microwave induction: Microwave power effects. Chem Eng Process 91:67–77
Park JH, Hwang RH, Yoon HC, Yi KB (2019) Effects of metal loading on activated carbon on its adsorption and desorption characteristics. J Ind Eng Chem 74:199–207
Chung TW, Chung CC (1999) Increase in the amount of adsorption on modified activated carbon by using neutron flux irradiation. Chem Eng Sci 54(12):1803–1809
Wang ZW, Xiao DT, Deng XY, Luo SH, Guo LF (2021) Liquid nitrogen modification method for improving radon adsorption efficiency of activated carbon. At Energy Sci Technol 55(02):377–384 ((in Chinese))
Liu L, Deng X, Liao Y, Xiao D, Wang M (2021) Activated carbon/attapulgite composites for radon adsorption. Mater Lett 285:129177
Jin S, Zheng G, Yu J (2020) A micro freeze-thaw damage model of concrete with fractal dimension. Constr Build Mater 257:119434
Lövqvist L, Balieu R, Kringos N (2020) A thermodynamics-based model for freeze-thaw damage in asphalt mixtures. Int J Solids Struct 203:264–275
Zhang H, Meng X, Yang G (2020) A study on mechanical properties and damage model of rock subjected to freeze-thaw cycles and confining pressure. Cold Reg Sci Technol 174:103056
Gore D, Heiden E, Snape I, Nash G, Stevens G (2006) Grain size of activated carbon, and untreated and modified granular clinoptilolite under freeze-thaw: Applications to permeable reactive barriers. Polar Rec 42(2):121–126
Hui K, Song L, Yin Z, Song H, Wang Z, Gao W, Xuan L (2022) Freeze-thaw combined with activated carbon improves electrochemical dewaterability of sludge: analysis of sludge floc structure and dewatering mechanism. Environ Sci Pollut Res Int 29(14):20333–20346
Jia HL (2016) Theoretical Damage Models of Porous Rocks and Hard Jointed Rocks Subjected to Frost Action and Further Experimental Verifications. China University of Geosciences, Hubei ((in Chinese))
Bridgman PW (1913) Water, in the liquid and five solid forms, under pressure. Am Acad Arts Sci 47:441–558
Hallet B (2006) Why do freezing rocks break? Science 314(5802):1092–1093
Vlahou I, Worster MG (2010) Ice growth in a spherical cavity of a porous medium. J Glaciol 56(196):271–277
Everett DH (1961) The thermodynamics of frost damage to porous solids. Trans Faraday Soc 57:1541–1551
Scherer GW (1999) Crystallization in pores. Cement Concrete Res 29(8):1347–1358
Setzer MJ (2001) Micro-ice-lens formation in porous solid. J Colloid Interf Sci 243(1):193–201
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic−inorganic nanocomposite materials. Chem Mater 13(10):3169–3183
Acknowledgements
We acknowledge support by the National Natural Science Foundation of China (11975120, 11875165).
Author information
Authors and Affiliations
Contributions
HL: Methodology, Data curation, Formal analysis, Writing-Original Draft. DX: Writing—review & editing, Resources, Project administration, Funding acquisition. GZ: Writing—review & editing, Resources, Project administration, Funding acquisition. XD: Formal analysis, Supervision. BY: Formal analysis, Visualization.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that none of the work contained in this manuscript is published in any language or currently under consideration at any other journal, and there are no conflicts of interest to declare. All authors have contributed to, read, and approved this submitted manuscript in its current form. Our manuscript has also been edited by a native English-speaking expert to ensure its English is good enough for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Xiao, D., Zhao, G. et al. Preparation and characterization of a high-efficiency radon adsorbing material based on activated carbon modified by water immersion and freeze–thaw. J Radioanal Nucl Chem 331, 3125–3133 (2022). https://doi.org/10.1007/s10967-022-08345-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08345-7