Abstract
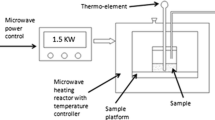
Activated carbon is a promising radon adsorbent. The desorption and recycling of activated carbon absorbed radon are of great significance to radon reduction and removal. In this paper, we proposed the closed-loop microwave desorption method of activated carbon. Specifically, we examined the percentage of mass loss, time and efficiency of desorption of the activated carbon absorbed radon in the microwave field. Our results show that the activated carbon loss percentage increases with the increase of gas flow rate and the mass loss percentage of activated carbon is lower in the closed-loop compared to it is in the open-loop desorption mode. The maximum cumulative loss percentage after nine consecutive desorption cycles of the activated carbon were 13.4 and 2.6% under the open- and closed-loop models, respectively. At the flow rate of 4, 8, 12 and 16 L min−1, in the closed-loop regeneration method, the static adsorption coefficients after nine adsorption–desorption cycles were 4.8, 6.1, 10.7 and 12.3% higher than that in the open-loop, respectively. The desorption efficiency of activated carbon adsorbed radon in the closed-loop method was 94.6–98.9%. Likewise, the activated carbon absorbed low 222Rn concentration (600 Bq m−3) can be desorbed completely in 45–50 min.











Similar content being viewed by others
References
NSCEAR (2000) Sources and effects of ionizing radiation. UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) 2000 Report to the General Assembly, with scientific annexes
Műllerová M, Mazur J, Blahušiak P, Grządziel D, Holý K, Kovács T, Kozak K, Csordás A, Neznal M, Neznal M, Shahrokhi A (2016) Indoor radon activity concentration in thermal spas: the comparison of three types of passive radon detectors. J Radioanal Nucl Chem 310(3):1077–1084
Ford Earl S, Eheman CR, Siegel PZ, Garbe PL (1996) Radon awareness and testing behavior. Health Phys 70(3):363–366
Ge XY, Tian F, Wu ZL, Yan YJ, Cravotto G, Wu ZS (2015) Adsorption of naphthalene from aqueous solution on coal-based activated carbon modified by microwave induction: microwave power effects. Chem Eng Process 91:67–77
Zhou QZ, Zhao GZ, Xiao DT, Qiu SK, Lei Q, Kearfott KJ (2018) Prediction of radon removal efficiency for a flow-through activated charcoal system and radon mitigation characteristics. Radiat Meas 119:112–120
Ania CO, Parra JB, Menéndez JA, Pis JJ (2005) Effect of microwave and conventional regeneration on the microporous and mesoporous network and on the adsorptive capacity of activated carbons. Microporous Mesoporous Mater 85(1–2):7–15
Bradshaw SM, Wyk EJ, De Swardt JB (1997) Microw Power Electromagn Energy 32:131
Price DW, Schmidt PS (1997) Microw Power Electromagn Energy 32:145
Grekov D, Pré P, Alappat BJ (2020) Microwave mode of heating in the preparation of porous carbon materials for adsorption and energy storage applications—an overview. Renew Sustain Energy Rev 124:109743
Menéndez JA, Arenillas A, Fidalgo B, Fernández Y, Zubizarreta L, Calvo EG, Bermúdez GM (2010) Microwave heating processes involving carbon materials. Fuel Process Technol 91(1):1–8
Yang H, Shan J, Li JL, Jiang ST (2019) Microwave desorption and regeneration methods for activated carbon with adsorbed radon. Adsorption 25(2):173–185
Liu QS, Wang P, Zhao SS, Zhang W (2012) Treatment of an industrial chemical waste-water using a granular activated carbon adsorption-microwave regeneration process. J Chem Technol Biotechnol 87(7):1004–1009
Gao J, Qi X, Zhang D, Matsuoka T, Nakamura Y (2020) Propagation of glowing combustion front in a packed bed of activated carbon particles and the role of co oxidation. Proc Combust Inst 000:1–10
Wang SN (2008) Study on the factors affecting the heating behavior of activated carbon in microwave field. Gansu Sci Technol 24(21):98–101+48 (in Chinese)
Wu RX (1982) A simple method for deriving Maxwell’s velocity distribution law. Coll Phys 09:7–11
He ZZ, Xiao DT, Zhao GZ, Qiu SK, Shan J, Fu Y, Ou YQ, Wu XJ (2013) Theoretical calculation and experimental determination of iterative correction factor for continuous measurement Radon method. At Energy Sci Technol 47(06):1040–1043 (in Chinese)
Wang ZW, Xiao DT, Deng XY, Luo SH, Guo LF (2021) Liquid nitrogen modification method for improving radon adsorption efficiency of activated carbon. At Energy Sci Technol 55(02):377–384 (in Chinese)
Acknowledgements
This study was supported by the National Natural Science Foundation of China (12075112).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, S., Shan, J., Li, S. et al. Use of microwave closed-loop desorption method to evaluate the desorption characteristics of activated carbon absorbed radon. J Radioanal Nucl Chem 331, 1785–1793 (2022). https://doi.org/10.1007/s10967-022-08243-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08243-y