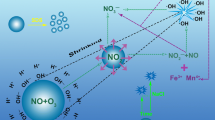
Abstract
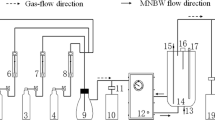
The oxidation efficiency and leaching rate of ozone micro-nano bubbles (OMNBs) on UO2 and sandstone uranium ore were explored and evaluated through a batch oxidation experiment of sandstone uranium ore and a continuous flow oxidation experiment of UO2. OMNBs can accelerate the rate of oxidative leaching. Oxidation mainly relies on ozone oxidation. Oxidation of sandstone uranium ore by OMNBs was feasible, and OMNBs may solve current problems encountered in production practice. The continuous flow experiment verified the feasibility of the large-scale experiment and provided a new method for CO2 + O2 ISL of uranium under normal pressure in the future.








Similar content being viewed by others
References
Falk J, Green J, Mudd G (2006) Australia, uranium and nuclear power. Int J Environ Stud 63(6):845–857. https://doi.org/10.1080/00207230601047131
Tulsidas H, Fairclough M (2018) World distribution of uranium deposits (UDEPO)
Filippov AP, Kanevskii EA (1965) Oxidation-reduction potentials and the degree of uranium leaching in sulphuric acid solutions. J Nucl Energy Parts A 19(7):575–580. https://doi.org/10.1016/0368-3230(65)90137-0
Mudd G (2001) Critical review of acid in situ leach uranium mining: 1 USA and Australia. Environ Geology 41(3–4):390–403. https://doi.org/10.1007/s002540100406
Mudd G (2001) Critical review of acid in situ leach uranium mining: 2. Soviet Block and Asia. Environ Geol 41(3–4):404–416. https://doi.org/10.1007/s002540100405
Ilankoon IMSK, Tang Y, Ghorbani Y, Northey S, Yellishetty M, Deng X, Mcbride D (2018) The current state and future directions of percolation leaching in the Chinese mining industry: challenges and opportunities. Miner Eng 125:206–222. https://doi.org/10.1016/j.mineng.2018.06.006
Xu D, Chi G, Nie F, Fayek M, Hu R (2021) Diversity of uranium deposits in China—an introduction to the special issue. Ore Geol Rev 129:103944. https://doi.org/10.1016/j.oregeorev.2020.103944
Alam MS, Cheng T (2014) Uranium release from sediment to groundwater: influence of water chemistry and insights into release mechanisms. J Contam Hydrol 164:72–87. https://doi.org/10.1016/j.jconhyd.2014.06.001
Bhargava SK, Ram R, Pownceby M, Grocott S, Ring B, Tardio J, Jones L (2015) A review of acid leaching of uraninite. Hydrometallurgy 151:10–24. https://doi.org/10.1016/j.hydromet.2014.10.015
Zhi M, Chang X, Que W, Niu Y, Wen Z, Wang H (2020) Experimental study on the application of Eluex process in acid ISL. J Radioanal Nucl Chem 325(2):551–556. https://doi.org/10.1007/s10967-020-07268-5
Robin V, Beaufort D, Tertre E, Reinholdt M, Fromaget M, Forestier S, de Boissezon H, Descostes M (2020) Fate of dioctahedral smectites in uranium roll front deposits exploited by acidic In Situ Recovery (ISR) solutions. Appl Clay Sci 187:105484. https://doi.org/10.1016/j.clay.2020.105484
Chung D-Y, Seo H-S, Lee J-W, Yang H-B, Lee E-H, Kim K-W (2010) Oxidative leaching of uranium from SIMFUEL using Na2CO3-H2O2 solution. J Radioanal Nucl Chem 284(1):123–129. https://doi.org/10.1007/s10967-009-0443-6
Kacham AR, Suri AK (2014) Application of a topochemical reaction model to predict leaching behavior of high carbonate uranium ores in alkaline solutions: an experimental case study. Hydrometallurgy 141:67–75. https://doi.org/10.1016/j.hydromet.2013.10.005
Asghar F, Sun Z, Chen G, Zhou Y, Li G, Liu H, Zhao K (2020) Geochemical characteristics and uranium neutral leaching through a CO2+O2 system—an example from uranium ore of the ELZPA ore deposit in Pakistan. Metals. https://doi.org/10.3390/met10121616
Asghar FZY, Sun ZX, Li GR, Xu LL, Zhao K (2021) CO2+O2 neutral leaching experiment of uranium ore from Mengqiguer deposit in Xinjiang. Nonferrous Metals Extra Metall 08:46–55. https://doi.org/10.3969/j.issn.1007-7545.2021.08.006 ((inChinese))
Xu LY, H.; Zhou Y, Li G (2020) Neutral leaching of uranium ore from a sandstone-type deposit. Nonferrous Metals Extra Metall 3:38–44. https://doi.org/10.3969/j.issn.1007-7545.2020.03.008(in Chinese)
Agarwal A, Ng WJ, Yu L (2011) Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 84(9):1175–1180. https://doi.org/10.1016/j.chemosphere.2011.05.054
Azevedo A, Oliveira H, Rubio J (2019) Bulk nanobubbles in the mineral and environmental areas: updating research and applications. Adv Colloid Interface Sci 271:101992. https://doi.org/10.1016/j.cis.2019.101992
Meegoda JN, Aluthgun Hewage S, Batagoda JH (2018) Stability of Nanobubbles. Environ Eng Sci 35(11):1216–1227. https://doi.org/10.1089/ees.2018.0203
Ghadimkhani A, Zhang W, Marhaba T (2016) Ceramic membrane defouling (cleaning) by air Nano Bubbles. Chemosphere 146:379–384. https://doi.org/10.1016/j.chemosphere.2015.12.023
Minamikawa K, Takahashi M, Makino T, Tago K, Hayatsu M (2015) Irrigation with oxygen-nanobubble water can reduce methane emission and arsenic dissolution in a flooded rice paddy. Environ Res Lett 10(8):084012. https://doi.org/10.1088/1748-9326/10/8/084012
Hu L, Xia Z (2017) Application of ozone micro-nano-bubbles to groundwater remediation. J Hazard Mater 342:446–453
Yamabe H, Nakaoka K, Xue Z, Matsuoka T, Kameyama H, Nishio S (2013) Simulation study of CO2 micro-bubble generation through porous media. Energy Procedia 37:4635–4646. https://doi.org/10.1016/j.egypro.2013.06.372
Kim Y, Oh J-I, Zhang M, Lee J, Park Y-K, Ho Lee K, Kwon EE (2019) Reduction of Na and K contents in bio-heavy oil using micro-/nano-sized CO2 bubbles. Journal of CO2 Utilization 34:430–436. doi:https://doi.org/10.1016/j.jcou.2019.07.031
Liu S, Oshita S, Thuyet DQ, Saito M, Yoshimoto T (2018) Antioxidant Activity of Hydrogen Nanobubbles in Water with Different Reactive Oxygen Species both in Vivo and in Vitro. Langmuir 34(39):11878–11885. https://doi.org/10.1021/acs.langmuir.8b02440
Xiao Z, Li D, Wang F, Sun Z, Lin Z (2020) Simultaneous removal of NO and SO2 with a new recycling micro-nano bubble oxidation-absorption process based on HA-Na. Sep Purif Technol 242:116788. https://doi.org/10.1016/j.seppur.2020.116788
Xiao Z, Li D, Zhu Q, Sun Z (2020) Simultaneous removal of NO and SO2 through a new wet recycling oxidation-reduction process utilizing micro-nano bubble gas-liquid dispersion system based on Na2SO3. Fuel 263:116882. https://doi.org/10.1016/j.fuel.2019.116682
Xiao Z, Li D, Zhang R, Wang F, Pan F, Sun Z (2020) An experimental study on the simultaneous removal of NO and SO2 with a new wet recycling process based on the micro-nano bubble water system. Environ Sci Pollut Res Int 27(4):4197–4205. https://doi.org/10.1007/s11356-019-07136-0
Xiao Z, Li D (2020) Simultaneous removal of NO and SO2 with a micro-bubble gas-liquid dispersion system based on air/H2O2/Na2S2O8. Environ Technol 41(27):3573–3583. https://doi.org/10.1080/09593330.2019.1615134
Li P, Wu C, Yang Y, Wang Y, Yu S, Xia S, Chu W (2018) Effects of microbubble ozonation on the formation of disinfection by-products in bromide-containing water from Tai Lake. Sep Purif Technol 193:408–414. https://doi.org/10.1016/j.seppur.2017.11.049
Khuntia S, Majumder SK, Ghosh P (2013) Removal of ammonia from water by ozone microbubbles. Ind Eng Chem Res 52(1):318–326. https://doi.org/10.1021/ie302212p
Ryskie S, Gonzalez-Merchan C, Neculita CM, Genty T (2020) Efficiency of ozone microbubbles for ammonia removal from mine effluents. Miner Eng 145:106071. https://doi.org/10.1016/j.mineng.2019.106071
Yang Z, Li Y, Ning Y, Yang S, Tang Y, Zhang Y, Wang X (2018) Effects of oxidant and particle size on uranium leaching from coal ash. J Radioanal Nucl Chem 317(2):801–810. https://doi.org/10.1007/s10967-018-5963-5
Pedroza FRC, Aguilar MDJS, Treviño TP, Luévanos AM, Castillo MS (2012) Treatment of sulfide minerals by oxidative leaching with ozone. Miner Process Extr Metall Rev 33(4):269–279. https://doi.org/10.1080/08827508.2011.584093
Ukasik M, Havlik T (2004) Effect of selected parameters on tetrahedrite leaching by ozone. Hydrometallurgy 77(1):139–145. https://doi.org/10.1016/j.hydromet.2004.10.017
Li Q, Li D, Qian F (2009) Pre-oxidation of high-sulfur and high-arsenic refractory gold concentrate by ozone and ferric ions in acidic media. Hydrometallurgy 97(1):61–66. https://doi.org/10.1016/j.hydromet.2009.01.002
Tian Q, Wang H, Xin Y, Li D, Guo X (2016) Ozonation leaching of a complex sulfidic antimony ore in hydrochloric acid solution. Hydrometallurgy 159:126–131. https://doi.org/10.1016/j.hydromet.2015.11.011
Fan W, An WG, Huo MX, Yang W, Zhu SY, Lin SS (2020) Solubilization and stabilization for prolonged reactivity of ozone using micro-nano bubbles and ozone-saturated solvent: a promising enhancement for ozonation. Sep Purif Technol 238:116484. https://doi.org/10.1016/j.seppur.2019.116484
Tocino F, Szenknect S, Mesbah A, Clavier N, Dacheux N (2014) Dissolution of uranium mixed oxides: the role of oxygen vacancies vs. the redox reactions. Prog Nucl Energy 72:101–106. https://doi.org/10.1016/j.pnucene.2013.09.014
Wang D, Yang X, Tian C, Lei Z, Kobayashi N, Kobayashi M, Adachi Y, Shimizu K, Zhang Z (2018) Characteristics of ultra-fine bubble water and its trials on enhanced methane production from waste activated sludge. Biores Technol 273:63–69. https://doi.org/10.1016/j.biortech.2018.10.077
Liu JH, Shi WJ, Liu YJ, Zhou YP, Sun ZX (2015) Comparative experiments on acid leaching and bioleaching to a sandstone type uranium ore. Adv Mater Res 1130:247–250. https://doi.org/10.4028/www.scientific.net/AMR.1130.247
Umanskii AB, Klyushnikov AM (2013) Bioleaching of low grade uranium ore containing pyrite using A. ferrooxidans and A. thiooxidans. J Radioanal Nucl Chem 295(1):151–156. https://doi.org/10.1007/s10967-012-1816-9
Tan K, Li C, Liu J, Qu H, Xia L, Hu Y, Li Y (2014) A novel method using a complex surfactant for in-situ leaching of low permeable sandstone uranium deposits. Hydrometallurgy 150:99–106. https://doi.org/10.1016/j.hydromet.2014.10.001
Du R, Zhang X, Li M, Wu X, Liu Y, Jiang T, Chen C, Peng Y (2019) Leaching of low permeable sandstone uranium ore using auxiliary materials: anionic surfactants. J Radioanal Nucl Chem 322(2):839–846. https://doi.org/10.1007/s10967-019-06793-2
Ulatowski K, Sobieszuk P, Mróz A, Ciach T (2019) Stability of nanobubbles generated in water using porous membrane system. Chem Eng Process 136:62–71. https://doi.org/10.1016/j.cep.2018.12.010
Zhou Z, Yang Z, Sun Z, Liu Y, Chen G, Liao Q, Xu L, Wang X, Li J, Zhou Y (2019) Enhanced uranium bioleaching high-fluorine and low-sulfur uranium ore by a mesophilic acidophilic bacterial consortium with pyrite. J Radioanal Nucl Chem 321(2):711–722. https://doi.org/10.1007/s10967-019-06608-4
Rodríguez-Rodríguez C, Nava-Alonso F, Uribe-Salas A (2014) Silver leaching from pyrargyrite oxidation by ozone in acid media. Hydrometallurgy 149:168–176. https://doi.org/10.1016/j.hydromet.2014.08.006
Nicol MJ, Needes CRS (1975) The anodic dissolution of uranium dioxide-I. in perchlorate solutions. Electrochimica Acta 20(8):585–589. https://doi.org/10.1016/0013-4686(75)80009-0
Ram R, Charalambous FA, McMaster S, Pownceby MI, Tardio J, Bhargava SK (2013) An investigation on the dissolution of natural uraninite ores. Miner Eng 50–51:83–92. https://doi.org/10.1016/j.mineng.2013.06.013
Li M, Huang C, Zhang X, Gao F, Wu X, Fang Q, Tan W, Zhang D (2018) Extraction mechanism of depleted uranium exposure by dilute alkali pretreatment combined with acid leaching. Hydrometallurgy 180:201–209. https://doi.org/10.1016/j.hydromet.2018.07.021
Khuntia S, Majumder SK, Ghosh P (2014) Oxidation of As(III) to As(V) using ozone microbubbles. Chemosphere 97:120–124. https://doi.org/10.1016/j.chemosphere.2013.10.046
Wang P, Hu E, Wang Q, Lei Z, Wang H, Zhang Y, Hou W, Zhang R (2019) Selective extraction of uranium from uranium–beryllium ore by acid leaching. J Radioanal Nucl Chem 322(2):597–604. https://doi.org/10.1007/s10967-019-06689-1
Shen N, Li J, Guo Y, Li X (2020) Thermodynamic modeling of in situ leaching of sandstone-type uranium minerals. J Chem Eng Data 65(4):2017–2031. https://doi.org/10.1021/acs.jced.9b01152
Pelalak R, Alizadeh R, Ghareshabani E, Heidari Z (2020) Degradation of sulfonamide antibiotics using ozone-based advanced oxidation process: experimental, modeling, transformation mechanism and DFT study. Sci Total Environ 734:139446. https://doi.org/10.1016/j.scitotenv.2020.139446
Anand Rao K, Sreenivas T, Vinjamur M, Suri AK (2014) Continuous leaching of uranium from an Indian ore: Residence time scale up and heat effects. Hydrometallurgy 146:119–127. https://doi.org/10.1016/j.hydromet.2014.03.014
Sheng Y, Li X, Deng W, Jiang F, Xing H, Wang R (2014) Risk analysis of gas supply system in CO2+O2 in-situ leaching uranium mining project based on HAZOP. Uranium Min Metall 129(1):8–12. https://doi.org/10.13426/j.cnki.yky.2014.01.003 ((inChinese))
Loh WH, Cai QQ, Li R, Jothinathan L, Hu JY (2021) Reverse osmosis concentrate treatment by microbubble ozonation-biological activated carbon process: organics removal performance and environmental impact assessment. Sci Total Environ 798:149289. https://doi.org/10.1016/j.scitotenv.2021.149289
Zhang R, Wang H, Hu E, Lei Z, Hu F, Hou W, Wang Q (2022) Oxidation of pyrite using ozone micro-nano bubbles. Min Metall Explor. https://doi.org/10.1007/s42461-021-00528-2
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (11675072), the Hunan Provincial Innovation Foundation for Postgraduate (CX20200906).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, R., Hou, W., Wang, H. et al. Oxidative leaching of sandstone uranium ore assisted by ozone micro-nano bubbles. J Radioanal Nucl Chem 331, 1645–1658 (2022). https://doi.org/10.1007/s10967-022-08241-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08241-0