Abstract
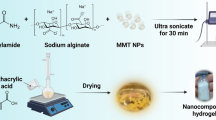
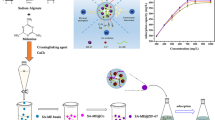
In this study, a novel type of composite hydrogel bead (PSH) was prepared by mixing of amidoximated polyacrylonitrile (PAO) and sodium alginate (SA). The structure of PSH was characterized with FT-IR, SEM and XPS. The influences of adsorption conditions on uranium(VI) adsorption by PSH were investigated by batch experiments. The kinetic and isotherm data were consistent with pseudo-second-order and Langmuir models, respectively. PSH displayed high selective adsorption capacity for uranium(VI). The maximum adsorption amount can reach 133.33 mg g−1. PSH was separated from aqueous solution easy and showed an excellent regeneration capacity. This research demonstrated that PSH had a broad prospect of application in the field of repairing uranium-containing wastewater.













Similar content being viewed by others
References
Barnham K, Mazzer M, Clive B (2006) Resolving the energy crisis: nuclear or photovoltaics? Nat Mater. https://doi.org/10.1038/nmat1604
Schiermeier Q, Tollefson J, Scully T et al (2008) Energy alternatives: electricity without carbon. Nature. https://doi.org/10.1038/454816a
Liu R, Wen S, Sun Y et al (2021) A nanoclay enhanced Amidoxime-Functionalized double-network hydrogel for fast and massive uranium recovery from seawater. Chem Eng J 2021:422. https://doi.org/10.1016/j.cej.2021.130060
Li FF, Cui WR, Jiang W et al (2020) Stable sp(2) carbon-conjugated covalent organic framework for detection and efficient adsorption of uranium from radioactive wastewater. J Hazard Mater 392:122333. https://doi.org/10.1016/j.jhazmat.2020.122333
Cui WR, Zhang CR, Xu RH et al (2021) Rational design of covalent organic frameworks as a groundbreaking uranium capture platform through three synergistic mechanisms. Appl Catal B 2021:294. https://doi.org/10.1016/j.apcatb.2021.120250
Broda E, Gładysz-Płaska A, Skwarek E et al (2021) Structural properties and adsorption of uranyl ions on the nanocomposite hydroxyapatite/white clay. Appl Nano. https://doi.org/10.1007/s13204-021-01790-y
Pan N, Tang J, Hou D et al (2021) Enhanced uranium uptake from acidic media achieved on a novel iron phosphate adsorbent. Chem Eng J 2021:423. https://doi.org/10.1016/j.cej.2021.130267
Wen Y, Yuan Y, Li L et al (2017) Ultrasensitive DNAzyme based amperometric determination of uranyl ion using mesoporous silica nanoparticles loaded with Methylene Blue. Mikrochim Acta 184(10):3909–3917. https://doi.org/10.1007/s00604-017-2397-7
Li L, Lu W, Ding D et al (2019) Adsorption properties of pyrene-functionalized nano-Fe3O4 mesoporous materials for uranium. J Solid State Chem 270:666–673. https://doi.org/10.1016/j.jssc.2018.12.030
Monier M, Elsayed NH (2014) Selective extraction of uranyl ions using ion-imprinted chelating microspheres. J Colloid Interface Sci 423:113–122. https://doi.org/10.1016/j.jcis.2014.02.015
Liu W, Zhao X, Wang T et al (2016) Adsorption of U(VI) by multilayer titanate nanotubes: effects of inorganic cations, carbonate and natural organic matter. Chem Eng J 286:427–435. https://doi.org/10.1016/j.cej.2015.10.094
Gan Q, Xu M, Li Q et al (2021) Two-dimensional ion-imprinted silica for selective uranium extraction from low-level radioactive effluents. ACS Sustain Chem Eng 9(23):7973–7981. https://doi.org/10.1021/acssuschemeng.1c02248
Liu C, Hsu PC, Xie J et al (2017) A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat Energy 2:4. https://doi.org/10.1038/nenergy.2017.7
Zhang S, Li H, Wang S (2020) Construction of an ion pathway boosts uranium extraction from seawater. Chem 6(7):1504–1505. https://doi.org/10.1016/j.chempr.2020.06.023
Amaral JCBS, Morais CA (2010) Thorium and uranium extraction from rare earth elements in monazite sulfuric acid liquor through solvent extraction. Miner Eng 23(6):498–503. https://doi.org/10.1016/j.mineng.2010.01.003
Suresh P, Duval CE (2020) Poly(acid)-functionalized membranes to sequester uranium from seawater. Ind Eng Chem Res 59(26):12212–12222. https://doi.org/10.1021/acs.iecr.0c01090
Amphlett JTM, Choi S, Parry SA et al (2020) Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: chelation vs. anion exchange. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123712
He N, Li H, Cheng C et al (2020) Enhanced marine applicability of adsorbent for uranium via synergy of hyperbranched poly(amido amine) and amidoxime groups. Chem Eng J 2020:395. https://doi.org/10.1016/j.cej.2020.125162
Wang S, Wang L, Li Z et al (2021) Highly efficient adsorption and immobilization of U(VI) from aqueous solution by alkalized MXene-supported nanoscale zero-valent iron. J Hazard Mater 408:124949. https://doi.org/10.1016/j.jhazmat.2020.124949
Zhao B, Yuan L, Wang Y et al (2021) Carboxylated UiO-66 tailored for U(VI) and Eu(III) trapping: from batch adsorption to dynamic column separation. ACS Appl Mater Int 13(14):16300–16308. https://doi.org/10.1021/acsami.1c00364
Huang Z, Li Z, Zheng L et al (2017) Interaction mechanism of uranium(VI) with three-dimensional graphene oxide-chitosan composite: Insights from batch experiments, IR, XPS, and EXAFS spectroscopy. Chem Eng J. https://doi.org/10.1016/j.cej.2017.07.067
Wang Z, Liu H, Lei Z et al (2020) Graphene aerogel for photocatalysis-assist uranium elimination under visible light and air atmosphere. Chem Eng J. https://doi.org/10.1016/j.cej.2020.126256
Wang Z, Hu H, Huang L et al (2020) Graphene aerogel capsulated precipitants for high efficiency and rapid elimination of uranium from water. Chem Eng J. https://doi.org/10.1016/j.cej.2020.125272
Zhu W, Li L, Dai L et al (2018) Bioassembly of fungal hyphae/carbon nanotubes composite as a versatile adsorbent for water pollution control. Chem Eng J 2018:339. https://doi.org/10.1016/j.cej.2018.01.134
Asiabi H, Yamini Y, Shamsayei S (2018) Highly efficient capture and recovery of uranium by reusable layered double hydroxide intercalated with 2-mercaptoethanesulfonate. Chem Eng J. https://doi.org/10.1016/j.cej.2017.12.143
Tachibana Y, Tanaka M, Nogami M (2019) Crown ether-type organic composite adsorbents embedded in high-porous silica beads for simultaneous recovery of lithium and uranium in seawater. J Radioanal Nucl Chem 322(2):717–730. https://doi.org/10.1007/s10967-019-06792-3
Ma F, Gui Y, Liu P et al (2020) Functional fibrous materials-based adsorbents for uranium adsorption and environmental remediation. Chem Eng J 3:90. https://doi.org/10.1016/j.cej.2020.124597
Li H, He N, Cheng C et al (2020) Antimicrobial polymer contained adsorbent: a promising candidate with remarkable anti-biofouling ability and durability for enhanced uranium extraction from seawater. Chem Eng J. https://doi.org/10.1016/j.cej.2020.124273
Zhang P, Wang L, Huang Z et al (2020) Aryl diazonium-assisted amidoximation of MXene for boosting water stability and uranyl sequestration via electrochemical sorption. ACS Appl Mater Interfaces 12(13):15579–15587. https://doi.org/10.1021/acsami.0c00861
Wang L, Yuan L, Chen K et al (2016) Loading actinides in multilayered structures for nuclear waste treatment: the first case study of uranium capture with vanadium carbide MXene. ACS Appl Mater Int 8(25):16396–16403. https://doi.org/10.1021/acsami.6b02989
Yuan Y, Yang Y, Ma X et al (2018) Molecularly imprinted porous aromatic frameworks and their composite components for selective extraction of uranium ions. Adv Mater 30(12):e1706507. https://doi.org/10.1002/adma.201706507
Bai J, Ma X, Yan H et al (2020) A novel functional porous organic polymer for the removal of uranium from wastewater. Microporous Mesoporous Mater 306:110441. https://doi.org/10.1016/j.micromeso.2020.110441
Sun Q, Aguila B, Earl LD et al (2018) Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv Mater 30(20):e1705479. https://doi.org/10.1002/adma.201705479
Yu B, Ye G, Chen J et al (2019) Membrane-supported 1D MOF hollow superstructure array prepared by polydopamine-regulated contra-diffusion synthesis for uranium entrapment. Environ Pollut 253:39–48. https://doi.org/10.1016/j.envpol.2019.06.114
Yu Q, Yuan Y, Feng L et al (2020) Spidroin-inspired, high-strength, loofah-shaped protein fiber for capturing uranium from seawater. Angew Chem Int Ed 59(37):15997–16001. https://doi.org/10.1002/anie.202007383
Kou S, Yang Z, Sun F (2017) Protein hydrogel microbeads for selective uranium mining from seawater. ACS Appl Mater Int. https://doi.org/10.1021/acsami.6b15968
Yuan Y, Yu Q, Wen J et al (2019) Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber. Angew Chem Int Ed 58(34):11785–11790. https://doi.org/10.1002/anie.201906191
Xiao F, Cheng Y, Zhou P et al (2021) Fabrication of novel carboxyl and amidoxime groups modified luffa fiber for highly efficient removal of uranium(VI) from uranium mine water. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105681
Lu W, Tang S, Li L et al (2019) Adsorption and recovery of amidoxime modified nano-Fe3O4-Aspergillus niger for U(VI) from low concentration uranium solution. Nanosci Nanotech Lett 11(3):337–345. https://doi.org/10.1166/nnl.2019.2896
Li L, Hu N, Ding D et al (2015) Adsorption and recovery of U(vi) from low concentration uranium solution by amidoxime modified Aspergillus niger. RSC Adv 5(81):65827–65839. https://doi.org/10.1039/c5ra13516h
Khamirchi R, Hosseini-Bandegharaei A, Alahabadi A et al (2018) Adsorption property of Br-PADAP-impregnated multiwall carbon nanotubes towards uranium and its performance in the selective separation and determination of uranium in different environmental samples. Ecotox Environ Safe 150:136–143. https://doi.org/10.1016/j.ecoenv.2017.12.039
Zhang J, Zhang H, Liu Q et al (2019) Diaminomaleonitrile functionalized double-shelled hollow MIL-101 (Cr) for selective removal of uranium from simulated seawater. Chem Eng J 368:951–958. https://doi.org/10.1016/j.cej.2019.02.096
Wang T, Xu M, Han X et al (2019) Petroleum pitch-based porous aromatic frameworks with phosphonate ligand for efficient separation of uranium from radioactive effluents. J Hazard Mater 368:214–220. https://doi.org/10.1016/j.jhazmat.2019.01.048
Gao Y, Yuan Y, Ma D et al (2014) Removal of aqueous uranyl ions by magnetic functionalized carboxymethylcellulose and adsorption property investigation. J Nucl Mater 453(1–3):82–90. https://doi.org/10.1016/j.jnucmat.2014.06.028
Li L, Huang F, Yuan Y et al (2013) Preparation and sorption performance of magnetic 18-crown-6/Fe3O4 nanocomposite for uranium(VI) in solution. J Radioanal Nucl Ch 298(1):227–235. https://doi.org/10.1007/s10967-013-2443-9
Li M, Liu H, Chen T et al (2019) Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci Total Environ 651(Pt 1):1020–1028. https://doi.org/10.1016/j.scitotenv.2018.09.259
Cui H, Pan N, Fan W et al (2019) Ultrafast fabrication of gradient nanoporous all-polysaccharide films as strong, superfast, and multiresponsive actuators. Asv Funct Mater. https://doi.org/10.1002/adfm.201807692
Şenol ZM, Şimşek S, Özer A et al (2021) Application of kaolinite-based composite as an adsorbent for removal of uranyl ions from aqueous solution: kinetics and equilibrium study. J Radioanal Nucl Ch. https://doi.org/10.1007/s10967-021-08070-7
Şenol ZM, Şimşek S, Özer A et al (2021) Synthesis and characterization of chitosan-vermiculite composite beads for removal of uranyl ions: isotherm, kinetics and thermody-namics studies. J Radioanal Nucl Ch 327:159–173. https://doi.org/10.1007/s10967-020-07481-2
Şenol ZM (2021) A chitosan-based composite for adsorption of uranyl ions; mechanism, isothems, kinetics and thermodynamics. Int J Biol Macromol 183:1640–1648. https://doi.org/10.1016/j.ijbiomac.2021.05.130
Şenol ZM, Şimşek S, Mahmood A et al (2020) Insight from adsorption properties of Xylidyl Blue embedded hydrogel for effective removal of uranyl: experimental and theoretical approaches. Polym Test 88:106566. https://doi.org/10.1016/j.polymertesting.2020.106566
Zou Z, Wang L, Zhou Z et al (2021) Simultaneous incorporation of PTH(1–34) and nano-hydroxyapatite into Chitosan/Alginate Hydrogels for efficient bone regeneration. Bioact Mater 6(6):1839–1851. https://doi.org/10.1016/j.bioactmat.2020.11.021
Hao D, Huang Q, Wei W et al (2021) A reusable, separation-free and biodegradable calcium alginate/g-C3N4 microsphere for sustainable photocatalytic wastewater treatment. J Clean Prod 3:14. https://doi.org/10.1016/j.jclepro.2021.128033
Saheed IO, Oh WD, Suah FBM (2021) Chitosan modifications for adsorption of pollutants—a review. J Hazard Mater 408:124889. https://doi.org/10.1016/j.jhazmat.2020.124889
Sessarego S, Rodrigues SCG, Xiao Y et al (2019) Phosphonium-enhanced chitosan for Cr(VI) adsorption in wastewater treatment. Carbohyd Polym 211:249–256. https://doi.org/10.1016/j.carbpol.2019.02.003
Yang SC, Liao Y, Karthikeyan KG et al (2021) Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ Pollut 286:117324. https://doi.org/10.1016/j.envpol.2021.117324
Wang Y, Lin N, Gong Y et al (2021) Cu-Fe embedded cross-linked 3D hydrogel for enhanced reductive removal of Cr(VI): characterization, performance, and mechanisms. Chemosphere 280:130663. https://doi.org/10.1016/j.chemosphere.2021.130663
Ao C, Zhao J, Li Q et al (2020) Biodegradable all-cellulose composite membranes for simultaneous oil/water separation and dye removal from water. Carbohyd Polym 250:116872. https://doi.org/10.1016/j.carbpol.2020.116872
Ren J, Zhu Z, Qiu Y et al (2021) Enhanced adsorption performance of alginate/MXene/CoFe2O4 for antibiotic and heavy metal under rotating magnetic field. Chemosphere 284:131284. https://doi.org/10.1016/j.chemosphere.2021.131284
Mondal H, Karmakar M, Ghosh NN et al (2021) One-pot synthesis of sodium alginate-grafted-terpolymer hydrogel for As(III) and V(V) removal: In situ anchored comonomer and DFT studies on structures. J Environ Manag 294:112932. https://doi.org/10.1016/j.jenvman.2021.112932
Xi H, Jiang H, Zhao D et al (2021) Highly selective adsorption of phosphate from high-salinity water environment using MgO-loaded and sodium alginate-immobilized bentonite beads. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.127773
Cho E, Kim J, Park CW et al (2018) Chemically bound Prussian blue in sodium alginate hydrogel for enhanced removal of Cs ions. J Hazard Mater 360:243–249. https://doi.org/10.1016/j.jhazmat.2018.08.031
Jiang X, Wang H, Wang Q et al (2020) Immobilizing amino-functionalized mesoporous silica into sodium alginate for efficiently removing low concentrations of uranium. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.119162
Jing B, Chu J, Qin Z et al (2020) Synthesis of amidoximated polyacrylonitrile nanoparticle/graphene composite hydrogel for selective uranium sorption from saline lake brine. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123553
Singh S, Bajwa BS, Kaur I (2020) (Zn/Co)-zeolitic imidazolate frameworks: room temperature synthesis and application as promising U(VI) scavengers—a comparative study. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2020.10.012
Zhenyuan B, Qi L, Wang J et al (2020) Anti-biofouling and water-stable balanced charged metal organic framework-based polyelectrolyte hydrogels for extracting uranium from seawater. ACS Appl Mater Interfaces 2020:18012–18022. https://doi.org/10.1021/acsami.0c03007
Wenting L, Yangyi L, Yang B et al (2020) Anchoring ZIF-67 particles on amidoximerized polyacrylonitrile fibers for radionuclide sequestration in wastewater and seawater. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.122692
Xuejie G, Haocheng Y, Jun W et al (2020) A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122850
Yue L, Yingzhong H, Xiangke W et al (2021) Impact of metal ions and organic ligands on uranium removal properties by zeolitic imidazolate framework materials. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.123216
Şimşek S, Şenol ZM, Ulusoy Hİ (2017) Synthesis and characterization of a composite polymeric material including chelating agent for adsorption of uranyl ions. J Hazard Mater 338:437–446. https://doi.org/10.1016/j.jhazmat.2017.05.059
Chongxiong D, Yi Z, Haochuan L et al (2020) Rapid room-temperature preparation of hierarchically porous metal-organic frameworks for efficient uranium removal from aqueous solutions. Nanomaterials 10(8):1539. https://doi.org/10.3390/nano10081539
Douchao M, Lijia L, Hongxing D et al (2022) Efficient uranium adsorbent with antimicrobial function constructed by grafting amidoxime groups on ZIF-90 via malononitrile intermediate. J Hazard Mater 42:2. https://doi.org/10.1016/j.jhazmat.2021.126872
Yuzhi Z, Dongxue L, Chang L et al (2021) Adsorption optimization of uranium(VI) onto polydopamine and sodium titanate co-functionalized MWCNTs using response surface methodology and a modeling approach. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2021.127145
Şenol ZM, Gül ÜD, Şimşek S (2021) Bioremoval of Safranin O dye by the identified lichen species called Evernia prunastri biomass: biosorption optimization, isotherms, kinetics, and thermodynamics. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01216-9
Şenol ZM, Şenol Arslan D, Şimşek S (2019) Preparation and characterization of a novel diatomite-based composite and investigation of its adsorption properties for uranyl ions. J Radioanal Nucl Ch 321:791–803. https://doi.org/10.1007/s10967-019-06662-y
Dai ZR, Zhang H, Sui Y et al (2018) Preparation of polyamidoxime/magnetic graphene oxide composite and its application for efficient extraction of uranium(VI) from aqueous solutions in an ultrasonic field. J Chem Eng Data 63(11):4215–4225. https://doi.org/10.1021/acs.jced.8b00703
Yin ZL, Xiong J, Chen M et al (2016) Recovery of uranium(VI) from aqueous solution by amidoxime functionalized wool fibers. J Radioanal Nucl Ch 307:1471–1479. https://doi.org/10.1007/s10967-015-4534-2
Zhang HJ, Zhang LX, Han XL et al (2018) Guanidine and amidoxime cofunctionalized polypropylene nonwoven fabric for potential uranium seawater extraction with antifouling property. Ind Eng Chem Res 57(5):1662–1670. https://doi.org/10.1021/acs.iecr.7b04687
Wang XL, Zhou JB, Zhang Z et al (2021) Synthesis of PAO NFs and the adsorption for uranium (VI) in alkaline solution. J Radioanal Nucl Ch. https://doi.org/10.1007/s10967-021-08083-2
Acknowledgements
This research was supported by the National Natural Science Foundation of China (12175103), the Key R&D Program of Hunan Province (2018SK2029), the Hunan Provincial Natural Science Foundation for Excellent Young Scholars (2020JJ3028), and the Scientific Research Innovation Project for Graduate of Hunan Province (CX20200913), Scientific Research Fund of Hunan Provincial Education Department (21A0259).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, B., Li, L., Dai, Z. et al. Synthesis of amidoximated polyacrylonitrile/sodium alginate composite hydrogel beed and its use in selective and recyclable removal of U(VI). J Radioanal Nucl Chem 331, 1669–1682 (2022). https://doi.org/10.1007/s10967-022-08233-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08233-0