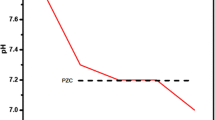
Abstract
A modified electrokinetic system using activated carbon particles was proposed to quickly remove uranium (U). The influences of electrokinetic parameters such as voltage, length of particle electrode, particle electrode ration were studied through batch experiments. The results revealed that the three dimensional electrokinetic (3D-EK) treatment system showed a great potential for uranium removal. The uranium removal efficiency reached above 99% and the residual solution reached the discharge standard when pH = 2, voltage = 25 V,150 g/L of 4 mm particle electrode. The colloidal precipitates generated were detected as Fe(OH)3. Tthe probable removal mechanism is ascribed to the synergy of electromigration, electrocoagulation and adsorption.














Similar content being viewed by others
References
Jingjing W, Bihong H, Xiaoyan W, Ping L (2019) Sorption of uranyl ions on TiO2: Effects of pH, contact time, ionic strength, temperature and HA. J Environ Sci (China) 75:115–123
Jianchao H, Zhongfu T, Jianhui W, Pinjie X (2011) Government policy and future projection for nuclear power in China. J Energ Eng, 151–159.
Wang WJ, Guan D, Tian YH, Liu Y, Zhu Y, He X (2019) Research progress in nuclear industry wastewater treatment. Nucl Eng Technol, 7(2).
Rahman ROA, Ibrahium HA, Hung Y (2011) Liquid radioactive wastes treatment: a review. Water 3(2):551–565
Landa ER (2004) Uranium mill tailings: nuclear waste and natural laboratory for geochemical and radioecological investigations. J Environ Radioact 77(1):1–27
Wen X (2000) Gulf War syndrome depleted uranium ammunition is the culprit. Foreign nuclear news 9
Cui P, Yunhai L, Xiaohong C, Min L, Goulin H, Rong H, Caixia W, Yating L, Xiaofu A (2011) Biosorption of uranium(VI) from aqueous solution by dead fungal biomass of Penicillium citrinum. Chem Eng J 170(1):1–6
Hongfu W, Faqin D, Mulan C, Wei Z, Miao H, Mingxue L (2020) Removal of uranium by biogenetic jarosite coupled with photoinduced reduction in the presence of oxalic acid: a low-cost remediation technology. J Radioanal Nucl Chem 324(2):715–729
Gyenam K, Wonsu K, Seungsu K, Ukryang P, Hyemin P, Jeikwon M (2012) Remediation of soil/concrete contaminated with uranium and radium by biological method. J Radioanal Nucl Chem 297(1):71–78
Ye D, Ping Z, Yujia Q, Qichao T, Yunfeng Y, Zhili H (2016) Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ Microbiol 18(1):205–218
Bin PS, Wook CD, Chan HS, Guk KJ, Hansoo L, Sik WM, Ho KY (2010) Investigation of the evaporation of rare earth chlorides in a LiCl–KCl molten salt. J Radioanal Nucl Chem 287(2):603–608
Fatemeh S, Zahra A, Mohamad S, Hamid S (2012) Uranium(VI) sorption behavior onto amberlite CG-400 anion exchange resin: Effects of pH, contact time, temperature and presence of phosphate. Ann Nucl Energy 48:21–24
Anirudhan TS, Radhakrishnan PG (2009) Improved performance of a biomaterial-based cation exchanger for the adsorption of uranium(VI) from water and nuclear industry wastewater. J Environ Radioact 100(3):250–257
Dimiropoulos V, Katsoyiannis IA, Zouboulis AI, Noli F, Simeonidis K, Mitrakas M (2015) Enhanced U(VI) removal from drinking water by nanostructured binary Fe/Mn oxy-hydroxides. J Water Process Eng 7:227–236
Duff MC, Coughlin JU, Hunter DB (2002) Uranium co-precipitation with iron oxide minerals. Geochim Cosmochim Ac 66:3533–3547
Lee M, Yang M (2010) Rhizofiltration using sunflower (Helianthus annuus L.) and bean (Phaseolus vulgaris L. var. vulgaris) to remediate uranium contaminated groundwater. J Hazard Mater 173:589–596
Bingqing L, Mi L, Xiaowen Z, Chunmei H, Xiaoyan W, Fang Q (2018) Immobilization of uranium into magnetite from aqueous solution by electrodepositing approach. J Hazard Mater 343:255–265
Xiaokun Y, Junya Z, Yan Z, Yuancai L, Rongni D, Shulong W, Lianghao L, Yuancai C, YongYou H (2016) Treatment of Ni-EDTA containing wastewater by electrocoagulation using iron scraps packed-bed anode. Chemosphere 164:304–313
Kamil OM, Hanife S-E (2018) Treatment of metal plating wastewater using iron electrode by electrocoagulation process: Optimization and process performance. Process Saf Environ Protection 119:207–217
Xiujuan C, Panpan R, Tao L, Jason PT, Xingbo L (2018) Zinc removal from model wastewater by electrocoagulation: Processing, kinetics and mechanism. Chem Eng J 349:358–367
Longqian X, Xiaojun X, Guangzhu C, Shuli L, Zhengyang D, Shumin S, Mingyao S, Mengjiao Z (2018) Optimization and assessment of Fe-electrocoagulation for the removal of potentially toxic metals from real smelting wastewater. J Environ Manage 218:129–138
Fu C, Xiaoxiao L, Zhanbin L, Jing M, Qianlin Z, Shaoliang Z (2018) Advanced treatment of copper smelting wastewater by the combination of internal micro-electrolysis and electrocoagulation. Sep Sci Technol 53(16):2639–2646
Mi L, Fangying G, Xiaowen Z, Shaoyan L, Jing H, Xiaoyan W, Qi F (2020) Recovery of uranium from low-grade tailings by electro-assisted leaching. J Clean Prod 271:122639
Shuibo X, Chun Z, Xinghuo Z, Jing Y, Xiaojian Z, Jingsong W (2009) Removal of uranium (VI) from aqueous solution by adsorption of hematite. J Environ Radioact 100(2):162–166
Tao H, Longfei L, Lulu Z, Shuwen Z (2018) Electrokinetic removal of chromium from chromite ore-processing residue using graphite particle-supported nanoscale zero-valent iron as the three-dimensional electrode. Chem Eng J 350:1022–1034
Jianwei M, Fayuan W, Zhenghong H, Hui W (2010) Simultaneous removal of 2,4-dichlorophenol and Cd from soils by electrokinetic remediation combined with activated bamboo charcoal. J Hazard Mater 176(1–3):715–720
Jiang X, Zhaohui P, Shukui Z, Luping C, Lishan R, Yingjiu L, Jiali L, Linyu T (2020) The mechanism of acid-washed zero-valent iron/activated carbon as permeable reactive barrier enhanced electrokinetic remediation of uranium-contaminated soil. Sep Purif Technol 244:116667
Tao H, Shuwen Z, Longfei L (2019) Immobilization of trace heavy metals in the electrokinetics-processed municipal solid waste incineration fly ashes and its characterizations and mechanisms. J Environ Manage 232:207–218
Yujie Y, Huilin L, Xu Y, Siyi L (2019) Efficient removal of chromium from soil in a modified electrokinetic system using iron-treated activated carbon as third electrode. J Taiwan Inst Chem E 101:15–23
Chao Z, Yonghai J, Yunlin L, Zhongxin H, Lei Z, Minghua Z (2013) Three-dimensional electrochemical process for wastewater treatment: A general review. Chem Eng J 228:455–467
Martínez SA, Rodríguez MG, Barrera C (2000) A kinetic model that describes removal of chromium VI from rinsing waters of the metal finishing industry by electrochemical processes. Water Sci Technol 42(5–6):55–61
Xiaohong L, Yuanyuan W, Shu L, Tianying Y (2015) Effects of anion on the electric double layer of imidazolium-based ionic liquids on graphite electrode by molecular dynamics simulation. Electrochim Acta 184:164–170
Yang KL, Ying TY, Yiacoumi S, Tsouris C, Vittoratos ES (2001) Electrosorption of ions from aqueous solutions by carbon aerogel: an electrical double-layer model. Langmuir 17:1961–1969
Mollah MYA, Schennach R, Parga JR, Cocke DL (2001) Electrocoagulation (EC) - science and application. J Hazard Mater 84:29–41
Yujie Y, Fengjiao X, Faheem M, Lin Y, Feng X, Binquan J, Shiau Y, Dongwei L (2018) Application of iron-loaded activated carbon electrodes for electrokinetic remediation of chromium-contaminated soil in a three-dimensional electrode system. Sci Rep 8(1):5753
Liao TQ, Feng TY, Li J, Hu JM, Yang LF, Zhang LB (2021) Pilot-scale removal of uranium from uranium plant wastewater using industrial iron powder in the ultrasonic field. Ann Nucl Energy 50:1–7
Wu XY, Lv CX, Yu SF, Li M, Ye J, Zhang XW, Liu Y (2020) Uranium (VI) removal from aqueous solution using iron-carbon micro-electrolysis packing. Sep Purif Technol 234:116104
Roshani M, Mirjalili K (2009) Studies on the leaching of an arseniceuranium ore. Hydrometallurgy 98(3):304–307
Susan AC, Grant D, Kliti G, JohnW M (2016) Uranium mobility in organic matter-rich sediments: A review of geological and geochemical processes. Earth-Sci Rev 159:160–185
Kim YS, Zeitlin H (1971) Separation of uranium from seawater by adsorbing colloid flotation. Anal Chem 43(11):1390–1393
Ulrich KU, Rossberg A, Foerstendorf H, Zanker H, Scheinost AC (2006) Molecular characterization of uranium(VI) sorption complexes on iron(III)-rich acid mine water colloids. Geochim Cosmochim Ac 70(22):5469–5487
Lee SY, Baik MH, Lee YJ, Lee YB (2009) Adsorption of U(VI) ions on biotite from aqueous solutions. Appl Clay Sci 46:255–259
Matthias T, Katsumi K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069
Hui T, Lei P, Xianming X, Wilkins RWT, Zhaoping M, Baojia H (2013) A preliminary study on the pore characterization of Lower Silurian black shales in the Chuandong thrust fold belt, southwestern China using low pressure N2 adsorption and FE-SEM methods. Mar Petrol Geol 48:8–19
Schimmack W, Gerstmann U, Schultz W, Geipel G (2007) Long-term corrosion and leaching of depleted uranium (DU) in soil. Radiat Environ Bioph 46(3):221–227
García-Gutiérrez M, Missana T, Fernńdez V (2003) Uranium (VI) sorption on colloidal magnetite under anoxic environment: experimental study and surface complexation modelling. Geochim Cosmochim Ac 67(14):2543–2550
Acknowledgements
The authors would like to thank the National Key Research and Development Program of China (2021YFC2902104), the National Natural Science Foundation of China (51974163), and the Key Scientific Research Foundation of Hunan Provincial Education Department (18A248), and he Natural Science Foundation of Hunan Province (Grants No. 2020JJ5494), the research project of education department of Hunan province(Grants No. 18C0439), and the initial Funds for the Universities of South China (Grants No.190XQD073) .
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, G., Wu, X., Zhang, S. et al. Efficient removal of uranium from aqueous solution in a modified three-dimensional electrokinetic system. J Radioanal Nucl Chem 331, 1585–1599 (2022). https://doi.org/10.1007/s10967-022-08225-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08225-0