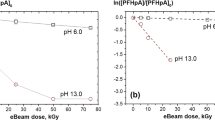
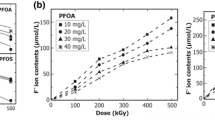
Abstract
Perfluorinated and hydrocarbon surfactants are important contaminants in wastewater. In this study, degradation of surfactants including PFOS, LAS, and CTAB under electron beam irradiation is investigated under different irradiation conditions (0–10 kGy absorbed dose, pH 3–11). Fulvic acid and H2O2 are used as active species inhibitors to study the effect of OH and eaq− on surfactants degradation. Irradiation degradation products of surfactants are analyzed by liquid chromatography-mass spectrometry. The degradation mechanisms of surfactants are summarized according to the experimental results. It shows that surfactants degradation is promoted at a high absorbed dose and pH value. The degradation rate of PFOS, LAS, and CTAB is 93.8%, 89.1%, and 80.6% maximum, respectively. The eaq− plays a dominant role in the breakage of C–C bonds in surfactants. The bonds of C–F, C–S, and C–N are mainly destroyed by OH. The degradation of surfactants follows pseudo-first-order kinetics. Degradation products of surfactants include various long and short-chain molecules. Furthermore, concentrations of short-chain products increase with absorbed dose because of further degradation of long-chain products. This study shows the potential application for electron beam irradiation technology to remove perfluorinated and hydrocarbon surfactants from wastewater.








Similar content being viewed by others
References
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40:3463–3473
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35:1339–1342
Liu DD, Xiu ZM, Liu F, Wu G, Adamson D, Newell C, Vikesland P, Tsai AL, Alvarez PJ (2013) Perfluorooctanoic acid degradation in the presence of Fe(III) under natural sunlight. J Hazard Mater 262:456–463
Mahinroosta R, Senevirathna L (2020) A review of the emerging treatment technologies for PFAS contaminated soils. J Environ Manag 255:109896
Moriwaki H, Takagi Y, Tanaka M, Tsuruho K, Okitsu K, Maeda Y (2005) Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ Sci Technol 39:3388–3392
Zhou Q, Deng SB, Yu Q, Zhang QY, Yu G, Huang J, He HP (2010) Sorption of perfluorooctane sulfonate on organo-montmorillonites. Chemosphere 78:688–694
Pan M-M, Li Q, Xu L (2020) Efficient adsorption of perfluoroalkyl acids by the quaternized hierarchically porous polystyrene-divinylbenzene. Chem Eng J 386:123990
Cheng HF, Sabatini DA (2002) Simultaneous uptake of anionic surfactants and micellar-solubilized contaminants using anion-exchange resins. Water Res 36:2062–2076
Jin L, Zhang P, Shao T, Zhao S (2014) Ferric ion mediated photodecomposition of aqueous perfluorooctane sulfonate (PFOS) under UV irradiation and its mechanism. J Hazard Mater 271:9–15
Kishimoto N, Doda K (2018) Effects of pH and coexisting chemicals on photolysis of perfluorooctane sulfonate using an excited xenon dimer lamp. Water Sci Technol 77:108–113
Levichev N, Lagunova Y, Seliverstov AF, Kireev SG, Tumashevich K, Shashkovskiy SG, Ershov BG (2020) Photodecomposition of sodium dodecyl sulfate under high-intensity pulsed UV radiation of continuous spectrum and hydrogen peroxide. Desalin Water Treat 196:131–136
Trojanowicz M, Bojanowska-Czajka A, Bartosiewicz I, Kulisa K (2018) Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) - a review of recent advances. Chem Eng J 336:170–199
Singla R, Grieser F, Ashokkumar M (2009) Kinetics and mechanism for the sonochemical degradation of a nonionic surfactant. J Phys Chem A 113:2865–2872
Hori H, Nagaoka Y, Murayama M, Kutsuna S (2008) Efficient decomposition of perfluorocarboxylic acids and alternative fluorochemical surfactants in hot water. Environ Sci Technol 42:7438–7443
Lee YC, Lo SL, Chiueh PT, Liou YH, Chen ML (2010) Microwave-hydrothermal decomposition of perfluorooctanoic acid in water by iron-activated persulfate oxidation. Water Res 44:886–892
Kim T-H, Lee S-H, Kim HY, Doudrick K, Yu S, Kim SD (2019) Decomposition of perfluorooctane sulfonate (PFOS) using a hybrid process with electron beam and chemical oxidants. Chem Eng J 361:1363–1370
Kowald C, Brorman E, Shankar S, Klemashevich C, Staack D, Pillai SD (2021) PFOA and PFOS breakdown in experimental sand, laboratory-grade water, investigation-derived groundwater and wastewater effluent samples at 50 kGy electron beam dose. Radiat Phys Chem 180:109323
Petrovic M, Gehringer P, Eschweiler H, Barcelo D (2007) Radiolytic decomposition of multi-class surfactants and their biotransformation products in sewage treatment plant effluents. Chemosphere 66:114–122
Getoff N (1996) Radiation-induced degradation of water pollutants—state of the art. Radiat Phys Chem 47:581–593
Selambakkannu S, Othman NAF, Abu Bakar K, Thailan KM, Karim Z (2021) Degradation of surfactants from domestic laundry effluent by electron beam irradiation. Mater Today Proc 46:1807–1812
Borrely SI, Romanelli MF, Pereira MCC, da Silva GP, Mesquita LCA, de Moraes MCF (2009) Radiation processing of detergents and possible environmental benefits. Nukleonika 54:61–64
Kim T-H, Yu S, Choi Y, Jeong T-Y, Kim SD (2018) Profiling the decomposition products of perfluorooctane sulfonate (PFOS) irradiated using an electron beam. Sci Total Environ 631–632:1295–1303
Ma S-H, Wu M-H, Tang L, Sun R, Zang C, Xiang J-J, Yang X-X, Li X, Xu G (2017) EB degradation of perfluorooctanoic acid and perfluorooctane sulfonate in aqueous solution. Nucl Sci Tech 28:1–8
Han B, Ko J, Kim J, Kim Y, Chung W, Makarov IE, Ponomarev AV, Pikaev AK (2002) Combined electron-beam and biological treatment of dyeing complex wastewater. Pilot plant Exp Radiat Phys Chem 64:53–59
Wang Y, Zhang PY, Pan G, Chen H (2008) Ferric ion mediated photochemical decomposition of perfluorooctanoic acid (PFOA) by 254 nm UV light. J Hazard Mater 160:181–186
Deng Y, Liang Z, Lu X, Chen D, Li Z, Wang F (2021) The degradation mechanisms of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) by different chemical methods: a critical review. Chemosphere 283:131168–131168
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (21771045), the project of Young Talents of China National Nuclear Corporation, University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2018013), Heilongjiang Provincial Postdoctoral Science Foundation (No. LBH-Z18232). The authors would like to thank Heilongjiang Institute of Atomic Energy for irradiation experiments and measurement.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiao, C., Men, X., Li, Z. et al. Degradation of representative perfluorinated and hydrocarbon surfactants by electron beam irradiation. J Radioanal Nucl Chem 331, 1691–1699 (2022). https://doi.org/10.1007/s10967-022-08224-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08224-1