Abstract
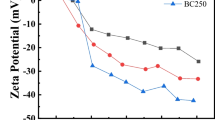
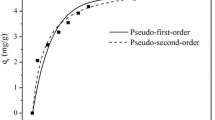
The removal of uranium from laboratory and environmental waters using oxidised biochar prepared from palm tree fibres has been investigated by means of batch type experiments. The effect of pH, contact time, temperature and initial uranium concentration has been studied and indicated that the adsorption follows the second order kinetic model and is an endothermic, entropy-driven process. According to the IR spectra the adsorption occurs via formation of inner-sphere complex formation between the surface carboxylic moieties and UO22+. The material has been effectively applied to remove uranium from groundwater, treated wastewater and seawater samples.








Similar content being viewed by others
References
Bleise A, Danesi PR, Bukart W (2003) Properties, use and health effects of depleted uranium (DU): a general overview. J Environ Radioact 64:93–112
Haneklaus N, Sun Y, Bol R, Lottermoser B, Schnug E (2017) To extract, or not to extract uranium from phosphate rock, that is the question. Environ Sci Technol 51:753–754
Aly MM, Hamza MF (2013) A review: studies on uranium removal using different techniques, overview. J Dispers Sci Technol 34:182–213
Zouboulis A, Katsoyiannis I (2013) Removal of uranium from contaminated drinking water: a mini review of available treatment methods. Desalin Water Treat 51:2915–2925
Hadjittofi L, Pashalidis I (2015) Uranium sorption from aqueous solutions by activated biochar fibres investigated by FTIR spectroscopy and batch experiments. J Radioanal Nucl Chem 302:897–904
Liatsou I, Michael G, Demetriou M, Pashalidis I (2017) Uranium binding by biochar fibres derived from Luffa cylindrical after controlled surface oxidation. J Radioanal Nucl Chem 311:871–875
Philippou K, Savva I, Pashalidis I (2018) Uranium(VI) binding by pine needles prior and after chemical modification. J Radioanal Nucl Chem 318(3):2205–2211
Chen TL, Kim H, Pan SY, Tseng PC, Lin YP, Chiang PC (2020) Implementation of green chemistry principles in circular economy system towards sustainable development goals: challenges and perspectives. Sci Total Environ 716:136998
Thompson KA, Shimabuku KK, Kearns JP, Knappe DR, Summers RS, Cook SM (2016) Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ Sci Technol 50:11253–11262
Liatsou I, Pashalidis I, Oezaslan M, Dosche C (2017) Surface characterization of oxidized biochar fibers derived from Luffa Cylindrica and lanthanide binding. J Environ Chem Eng 5:4069–4074
Liatsou I, Pashalidis I, Nicolaides A (2018) Triggering selective uranium separation from aqueous solutions by using salophen-modified biochar fibers. J Radioanal Nucl Chem 318:2199–2203
Philippou K, Anastopoulos I, Dosche C, Pashalidis I (2019) Synthesis and characterization of a novel Fe3O4-loaded oxidized biochar from pine needles and its application for uranium removal Kinetic, thermodynamic, and mechanistic analysis. J Environ Manage 252:109677
Ioannou K, Hadjiyiannis P, Liatsou I, Pashalidis I (2019) U(VI) adsorption by biochar fiber–MnO2 composites. J Radioanal Nucl Chem 320:425–432
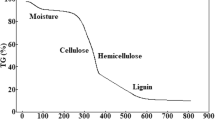
Reddy N, Yang Y (2015) Innovative Biofibers from Renewable Resources: Fibers from Palm Trees In: Natural Cellulose Fibers from Renewable Resources. Springer-Verlag, Berlin Heidelberg
Khan MH, Warwick P, Evans N (2006) Spectrophotometric determination of uranium with arsenazo-III in perchloric acid. Chemosphere 63:1165–1169
Kiliari T, Pashalidis I (2010) Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat Meas 45:966–968
Ishak MR, Leman Z, Sapuan SM, Rahman MZA, Anwar UMK (2013) Chemical composition and FT-IR spectra of sugar palm (Arenga pinnata) fibers obtained from different heights. J Nat Fibers 10(2):83–97
Hadjittofi L, Prodromou M, Pashalidis I (2014) Activated biochar derived from cactus fibres-Preparation, characterization and application on Cu(II) removal from aqueous solutions. Bioresource Technol 159:460–464
Pashalidis I, Czerwinski KR, Fanghaenel T, Kim JI (1997) A study of solid–liguid phase equilibria of Pu(VI) and U(VI) in aqueous carbonate systems. Determination of the carbonate stability constants. Radiochim Acta 76:55–62
Jin J, Li S, Peng X, Liu W, Zhang C, Yang Y, Han L, Du Z, Sun K, Wang X (2018) HNO3 modified biochars for uranium(VI) removal from aqueous solution. Bioresour Technol 256:247–253
Lagergen S (1898) About the theory of so-called adsorption of soluble substances. Handlingar 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stasi, C., Georgiou, E., Ioannidis, I. et al. Uranium removal from laboratory and environmental waters by oxidised biochar prepared from palm tree fibres. J Radioanal Nucl Chem 331, 375–381 (2022). https://doi.org/10.1007/s10967-021-08076-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-08076-1