Abstract
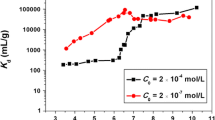
The adsorption characteristics of Eu(III) on colloidal bentonite particles were investigated by batch experiments as functions of colloid concentration, pH, foreign ions, and temperature. Bentonite colloids displayed remarkable adsorption ability to Eu(III), the Eu(III) adsorption was significantly affected by solution chemistry. The Eu(III) adsorption increased with colloids concentration and pH increasing. Divalent cations (Ca2+, Mg2+ and Sr2+) and anions (Cl− and SO42−) inhibited Eu(III) adsorption, whereas PO43− greatly enhanced Eu(III) adsorption. High temperature was beneficial for Eu(III) adsorption, the adsorption process was a spontaneous endothermic process. The results suggested that colloids could acted as an efficient carrier for Eu(III) transport.






Similar content being viewed by others
References
Geckeis H, Ngo Manh T, Bouby M, Kim JI (2003) Aquatic colloids relevant to radionuclide migration: characterization by size fractionation and ICP-mass spectrometric detection. Colloid Surfaces A 217(1–3):101–108
Nishad S, Al-Raoush RI (2021) Colloid retention and mobilization mechanisms under different physicochemical conditions in porous media: a micromodel study. Powder Technol 377:163–173
Ghiasi B, Niksokhan MH, Mahdavi Mazdeh A (2020) Co-transport of chromium(VI) and bentonite colloidal particles in water-saturated porous media: Effect of colloid concentration, sand gradation, and flow velocity. J Contam Hydrol 234:103682
Fan QH, Xu JZ, Niu ZW, Li P, Wu WS (2012) Investigation of Cs(I) uptake on Beishan soil combined batch and EDS techniques. Appl Radiat Isot 70(1):13–19
Chen Y, Zhu C, Sun Y, Duan H, Ye W, Wu D (2012) Adsorption of La(III) onto GMZ bentonite: effect of contact time, bentonite content, pH value and ionic strength. J Radioanal Nucl Chem 292(3):1339–1347
Patel MA, Kar AS, Kumar S, Das MK, Raut VV, Tomar BS (2018) Effect of sulfate on sorption of Eu(III) by Na-montmorillonite. Radiochim Acta 107:1–14
Rabung T, Pierret MC, Bauer A, Geckeis H, Bradbury MH, Baeyens B (2005) Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 1: batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim Cosmochim Ac 69(23):5393–5402
Songsheng L, Hua X, Mingming W, Xiaoping S, Qiong L (2011) Sorption of Eu(III) onto Gaomiaozi bentonite by batch technique as a function of pH, ionic strength, and humic acid. J Radioanal Nucl Chem 292(2):889–895
Pan DQ, Fan QH, Li P, Liu SP, Wu WS (2011) Sorption of Th(IV) on Na-bentonite: effects of pH, ionic strength, humic substances and temperature. Chem Eng J 172(2–3):898–905
Alonso U, Missana T, Patelli A, Rigato V (2007) Bentonite colloid diffusion through the host rock of a deep geological repository. Phy Chem Earth 32(1–7):469–476
Missana T, Garcı́a-Gutiérrez M, Alonso Ú (2004) Kinetics and irreversibility of cesium and uranium sorption onto bentonite colloids in a deep granitic environment. Appl Clay Sci 26(1–4):137–150
Degueldre C, Triay I, Kim J-I, Vilks P, Laaksoharju M, Miekeley N (2000) Groundwater colloid properties: a global approach. Appl Geochem 15(7):1043–1051
Bouby M, Geckeis H, Lützenkirchen J, Mihai S, Schäfer T (2011) Interaction of bentonite colloids with Cs, Eu, Th and U in presence of humic acid: a flow field-flow fractionation study. Geochim Cosmochim Acta 75(13):3866–3880
Zanker H, Hennig C (2014) Colloid-borne forms of tetravalent actinides: a brief review. J Contam Hydrol 157(1):87–105
Ye WM, Cui YJ, Qian LX, Chen B (2009) An experimental study of the water transfer through confined compacted GMZ bentonite. Eng Geol 108(3–4):169–176
Ye WM, Chen YG, Chen B, Wang Q, Wang J (2010) Advances on the knowledge of the buffer/backfill properties of heavily-compacted GMZ bentonite. Eng Geol 116(1–2):12–20
Baik MH, Cho WJ, Hahn PS (2007) Erosion of bentonite particles at the interface of a compacted bentonite and a fractured granite. Eng Geol 91(2–4):229–239
Xu Y, Gao Z, Chu F, Liu C (2016) Fractal model for erosion mass of bentonite colloids. Environ Earth Sci 75(19):1229–1238
Albarran N, Degueldre C, Missana T, Alonso U, García-Gutiérrez M, López T (2014) Size distribution analysis of colloid generated from compacted bentonite in low ionic strength aqueous solutions. Appl Clay Sci 95(4):284–293
Bessho K, Degueldre C (2009) Generation and sedimentation of colloidal bentonite particles in water. Appl Clay Sci 43(2):253–259
Missana T, Alonso U, Albarran N, García-Gutiérrez M, Cormenzana JL (2011) Analysis of colloids erosion from the bentonite barrier of a high level radioactive waste repository and implications in safety assessment. Phys Chem Earth 36(17–18):1607–1615
Elo O, Hölttä P, Kekäläinen P, Voutilainen M, Huittinen N (2019) Neptunium(V) transport in granitic rock: a laboratory scale study on the influence of bentonite colloids. Appl Geochem 103:31–39
Dittrich TM, Boukhalfa H, Ware SD, Reimus PW (2015) Laboratory investigation of the role of desorption kinetics on americium transport associated with bentonite colloids. J Environ Radioact 148(15):170–182
Albarran N, Missana T, Garcia-Gutierrez M, Alonso U, Mingarro M (2011) Strontium migration in a crystalline medium: effects of the presence of bentonite colloids. J Contam Hydrol 122(1–4):76–85
Wei X, Pan D, Xu Z, Xian D, Li X, Tan Z, Liu C, Wu W (2021) Colloidal stability and correlated migration of illite in the aquatic environment: the roles of pH, temperature, multiple cations and humic acid. Sci Total Environ 768:144174
Sun Y, Pan D, Wei X, Xian D, Wang P, Hou J, Xu Z, Liu C, Wu W (2020) Insight into the stability and correlated transport of kaolinite colloid: effect of pH, electrolytes and humic substances. Environ Pollut 266:115189
Walshe GE, Pang L, Flury M, Close ME, Flintoft M (2010) Effects of pH, ionic strength, dissolved organic matter, and flow rate on the co-transport of MS2 bacteriophages with kaolinite in gravel aquifer media. Water Res 44(4):1255–1269
Yang H, Ge Z, Wu D, Tong M, Ni J (2016) Cotransport of bacteria with hematite in porous media: effects of ion valence and humic acid. Water Res 88(1):586–594
Telfeyan K, Reimus PW, Boukhalfa H, Ware SD (2020) Aging effects on Cesium-137 (137Cs) sorption and transport in association with clay colloids. J Colloid Interface Sci 566:316–326
Li P, Liu Z, Ma F, Shi Q, Guo Z, Wu W (2015) Effects of pH, ionic strength and humic acid on the sorption of neptunium(V) to Na-bentonite. J Mol Liq 206(4):285–292
Hu J, Xie Z, He B, Sheng G, Chen C, Li J, Chen Y, Wang X (2010) Sorption of Eu(III) on GMZ bentonite in the absence/presence of humic acid studied by batch and XAFS techniques. Sci China Chem 53(6):1420–1428
Schnurr A, Marsac R, Rabung T, Lützenkirchen J, Geckeis H (2015) Sorption of Cm(III) and Eu(III) onto clay minerals under saline conditions: batch adsorption, laser-fluorescence spectroscopy and modeling. Geochim Cosmochim Acta 151(5):192–202
Tan XL, Wang XK (2008) Sorption of Eu(III) on humic acid or fulvic acid bound to hydrous alumina studied by SEM-EDS, XPS, TRLFS, and batch techniques. Environ Sci Technol 42(5):6532–6537
Bradbury MH, Baeyens B (2005) Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montmorillonite: linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochim Cosmochim Acta 69(4):875–892
Xu Z, Sun Y, Niu Z, Xu Y, Wei X, Chen X, Pan D, Wu W (2020) Kinetic determination of sedimentation for GMZ bentonite colloids in aqueous solution: effect of pH, temperature and electrolyte concentration. Appl Clay Sci 184:105393
Xu Z, Pan D, Sun Y, Wu W (2018) Stability of GMZ bentonite colloids: aggregation kinetic and reversibility study. Appl Clay Sci 161(1):436–443
Carstens JF, Bachmann J, Guggenberger G (2021) Aggregation and transport behavior of goethite colloids as affected by dissolved organic matter and pH: electrostatic vs. hydrophilic interactions. Colloid Surface A 609:125639
Praetorius A, Badetti E, Brunelli A, Clavier A, Gallego-Urrea JA, Gondikas A, Hassellöv M, Hofmann T, Mackevica A, Marcomini A, Peijnenburg W, Quik JTK, Seijo M, Stoll S, Tepe N, Walch H, von der Kammer F (2020) Strategies for determining heteroaggregation attachment efficiencies of engineered nanoparticles in aquatic environments. Environ Sci Nano 10:1039
Liang Y, Bradford SA, Simunek J, Klumpp E (2019) Mechanisms of graphene oxide aggregation, retention, and release in quartz sand. Sci Total Environ 656:70–79
Markus AA, Parsons JR, Roex EW, de Voogt P, Laane RW (2015) Modeling aggregation and sedimentation of nanoparticles in the aquatic environment. Sci Total Environ 506–507:323–329
Verma PK, Romanchuk AY, Vlasova IE, Krupskaya VV, Zakusin SV, Sobolev AV, Egorov AV, Mohapatra PK, Kalmykov SN (2017) Np(V) uptake by bentonite clay: effect of accessory Fe oxides/hydroxides on sorption and speciation. Appl Geochem 78:74–82
Verma PK, Pathak P, Mohapatra PK (2014) Sorption of metal cations on suspended bentonite: effects of pH, ionic strength and complexing anions. Radiochim Acta 102(5):401–409
Košak A, Bauman M, Padežnik-Gomilšek J, Lobnik A (2017) Lead(II) complexation with 3-mercaptopropyl-groups in the surface layer of silica nanoparticles: Sorption, kinetics and EXAFS/XANES study. J Mol Liq 229:371–379
Rihs S, Gaillard C, Reich T, Kohler SJ (2014) Uranyl sorption onto birnessite: a surface complexation modeling and EXAFS study. Chem Geol 373:59–70
Benedicto A, Missana T, Degueldre C (2013) Predictions of TiO2-driven migration of Se(IV) based on an integrated study of TiO2 colloid stability and Se(IV) surface adsorption. Sci Total Environ 449(1):214–222
Sø HU, Postma D, Jakobsen R, Larsen F (2011) Sorption of phosphate onto calcite; results from batch experiments and surface complexation modeling. Geochim Cosmochim Acta 75(10):2911–2923
Fan Q, Shao D, Lu Y, Wu W, Wang X (2009) Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni(II) to Na-attapulgite. Chem Eng J 150(1):188–195
Chavez ML, de Pablo L, Garcia TA (2010) Adsorption of Ba2+ by Ca-exchange clinoptilolite tuff and montmorillonite clay. J Hazard Mater 175(1–3):216–223
Missana T, Garcia-Gutierrez M, Alonso U (2008) Sorption of strontium onto illite/smectite mixed clays. Phy Chem Earth 33:S156–S162
Magzoub MI, Nasser MS, Hussein IA, Benamor A, Onaizi SA, Sultan AS, Mahmoud MA (2017) Effects of sodium carbonate addition, heat and agitation on swelling and rheological behavior of Ca-bentonite colloidal dispersions. Appl Clay Sci 147(2):176–183
Huang S, Pang H, Li L, Jiang S, Wen T, Zhuang L, Hu B, Wang X (2018) Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from MIL-53. Chem Eng J 353(6):157–166
Bachmaf S, Planer-Friedrich B, Merkel BJ (2008) Effect of sulfate, carbonate, and phosphate on the Uranium(VI) sorption behavior onto bentonite. Radiochim Acta 96(6):359–366
Xu L, Zheng T, Yang S, Zhang L, Wang J, Liu W, Chen L, Diwu J, Chai Z, Wang S (2016) Uptake mechanisms of Eu(III) on hydroxyapatite: a potential permeable reactive barrier backfill material for trapping trivalent minor actinides. Environ Sci Technol 50(7):3852–3829
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22006060, U1730245, 21806063); the Fundamental Research Funds for the Central Universities (lzujbky-2021-sp29, lzujbky-2020-kb06).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Z., Niu, Z., Tang, Q. et al. Adsorption characteristics of Eu(III) on colloidal bentonite particles in aqueous solution: impact of colloid concentration, pH, foreign ions, and temperature. J Radioanal Nucl Chem 330, 765–773 (2021). https://doi.org/10.1007/s10967-021-07976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07976-6