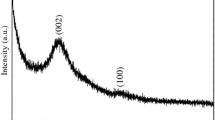
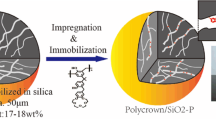
Abstract
In the present study, a porous silica-based covalent organic framework incorporated composite was prepared using a solvothermal reaction and the following wet-impregnation method. Multiple characterization methods including Scanning electron microscope, Fourier transform infrared spectroscopy, X-ray powder diffraction confirmed the surface morphology, thermal stability, and pore structure of the as-prepared composite. The adsorption performances of the prepared composite toward palladium [Pd(II)] from simulated high-level liquid waste were systematically investigated using batch experiments under the effect of contact time, solution temperature, and nitric acid solution.









Similar content being viewed by others
References
Sengupta P (2012) A review on immobilization of phosphate containing high level nuclear wastes within glass matrix–present status and future challenges. J Hazard Mater 235:23617–23628
Sant’ana LP, Cordeiro TC (2016) Management of radioactive waste: a review. Proc Int Acad Ecol Environ Sci 6:38–43
Abdel RRO, Ibrahium HA, Hung YT (2011) Liquid radioactive wastes treatment: a review. Water 3:551–565
Sood DD, Patil SK (1996) Chemistry of nuclear fuel reprocessing: current status. J Radioanal Nucl Chem 203:547–573
Smith RL Jr, Atmaji P, Hakuta Y, Kawaguchi M, Adschiri T, Arai K (1997) Recovery of metals from simulated high-level liquid waste with hydrothermal crystallization. J Supercrit Fluids 11:103–114
Bourg S, Poinssot C (2017) Could spent nuclear fuel be considered as a non-conventional mine of critical raw materials. Prog Nucl Energy 94:222–228
Schulz WW, Bray LA (1987) Solvent extraction recovery of byproduct 137Cs and 90Sr from HNO3 solutions–a technology review and assessment. Sep Sci Technol 22:191–214
Egorova KS, Ananikov VP (2016) Which metals are green for catalysis? comparison of the toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au salts. Angew Chem Int Ed 55:12150–12162
Sekimoto S, Nakagawa H, Okazaki S, Fukuda K, Asakura S, Shigemori T, Takahashi S (2000) A fiber-optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sens Actuators B Chem 66:142–145
Oh MY, Park SK, Park H, Kim H, Kang K, Kim JH, Roh KC, Shin TH (2018) Enhancement of oxygen reduction reaction catalytic activity via the modified surface of La0.6Sr0.4Co0.2Fe0.8O3−δ with palladium nanoparticles as cathode for lithium−air battery. ACS Appl Energy Mater 1:5518–5526
Reith F, Campbell SG, Ball AS, Pring A, Southam G (2014) Platinum in Earth surface environments. Earth Sci Rev 131:1–21
Cowley A (2021) The PGM market report. http://www.platinum.matthey.com/documents/new-item/pgm-market-reports/pgm-market-report-february-2021.pdf
Jensen GA, Platt AM, Mellinger GB, Bjorklund WJ (1984) Recovery of noble metals from fission products. Nucl Technol 65:305–324
Bush RP (1991) Recovery of platinum group metals from high level radioactive waste. Platin Metals Rev 35:202–208
Li FH, Shang Y, Ding ZM, Weng HQ, Xiao JX, Lin MZ (2017) Efficient extraction and separation of palladium (Pd) and ruthenium (Ru) from simulated HLLW by photoreduction. Sep Purif Technol 182:9–18
Ruhela R, Singh AK, Tomar BS, Hubli RC (2014) Separation of palladium from high level liquid waste–a review. RSC Adv 4:24344–24350
Pokhitonov YA (2020) Recovery of platinoids from NPP spent nuclear fuel and outlook for their use. At Energy 127:367–374
Ansari SA, Mohapatra PK (2017) A review on solid phase extraction of actinides and lanthanides with amide based extractants. J Chromatogr A 1499:1–20
Telmore VM, Kumar P, Jaison PG (2018) Study on complexation of palladium with thiourea-based ligands and its determination in simulated high-level liquid waste using solid phase extraction-electrospray mass spectrometry. J Radioanal Nucl Chem 318:1249–1259
Cote AP, Benin AI, Ockwig NW, O’Keeffe M, Matzger AJ, Yaghi OM (2005) Porous, crystalline, covalent organic frameworks. Science 310:1166
Corma A, Garcia H, Llabres i Xamena FX (2010) Engineering metal organic frameworks for heterogeneous catalysis. Chem Rev 110:4606–4655
Lohse MS, Bein T (2018) Covalent organic frameworks: structures, synthesis, and applications. Adv Funct Mater 28:1705553
Geng KY, He T, Liu RY, Dalapati S, Tan KT, Li ZP, Tao SS, Gong YF, Jiang QH, Jiang DL (2020) Covalent organic frameworks: design, synthesis, and functions. Chem Rev 120:8814–8933
Kandambeth S, Dey K, Banerjee R (2019) Covalent organic frameworks: chemistry beyond the structure. J Am Chem Soc 141:1807–1822
Kandambeth S, Mallick A, Lukose B, Mane MV, Heine T, Banerjee R (2012) Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J Am Chem Soc 134:19524–19527
Zhang J, Zhou LH, Jia ZM, Li XF, Qi Y, Yang CT, Guo XH, Chen SY, Long HH, Ma LJ (2020) Construction of covalent organic framework with unique double-ring pore for size-matching adsorption of uranium. Nanoscale 12:24044–24053
Lei ZD, Yang QS, Xu Y, Guo SY, Sun WW, Liu H, Lv LP, Zhang Y, Wang Y (2018) Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat Commun 9:576
Wen L, Wu P, Wang LL, Chen LZ, Wang ML, Wang X, Lin JM, Zhao RS (2020) Solid-phase microextraction using a β-ketoenamine-linked covalent organic framework coating for efficient enrichment of synthetic musks in water samples. Anal Methods 12:2434–2442
Yang CX, Liu C, Cao YM, Yan XP (2015) Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem Commun 51:12254–12257
Colson JW, Woll AR, Mukherjee A, Levendorf MP, Spitler EL, Shields VB, Spencer MG, Park J, Dichtel WR (2011) Oriented 2D covalent organic framework thin films on single-layer graphene. Science 332:228–231
You LJ, Xu K, Ding GJ, Shi XM, Li JM, Wang SY, Wang JB (2020) Facile synthesis of Fe3O4@COF covalent organic frameworks for the adsorption of bisphenols from aqueous solution. J Mol Liq 320:114456
Wu Y, Lee CP, Mimura H, Zhang XX, Wei YZ (2018) Stable solidification of silica-based ammonium molybdophosphate byallophane: application to treatment of radioactive cesium in secondary solid wastes generated from fukushima. J Hazard Mater 341:46–54
Tan J, Namuangruk S, Kong W, Kungwan N, Guo J, Wang CC (2016) Manipulation of amorphous-to-crystalline transformation: towards the construction of covalent organic framework hybrid microspheres with NIR photothermal conversion ability. Angew Chem Int Ed 55:13979–13984
Rabiul Awual Md, Khraisheh M, Alharthi NH, Luqman M, Islam A, Karim MR, Rahman MM, Khaleque MdA (2018) Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem Eng J 343:118–127
Wang HP, Wang T, Ma RR, Wu K, Li HL, Feng B, Li C, Shen YH (2020) Facile synthesis of sulfonated covalent organic framework for the adsorption of heavy metal ions. J Taiwan Inst Chem Eng 112:122–129
ALOthman ZA (2012) A review: fundamental aspects of silicate mesoporous materials. Mater 5:2874–2902
Vaibhav V, Vijayalakshmi U, Roopan SM (2015) Agricultural waste as a source for the production of silica nanoparticles. Spectrochim Acta A 139:515–520
Ranjbakhsha E, Bordbar AK, Abbasia M, Khosropoura AR, Shams E (2012) Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem Eng J 179:272–276
Wang W, Deng SB, Ren L, Li DY, Wang WJ, Vakili M, Wang B, Huang J, Wang YJ, Yu G (2018) Stable covalent organic frameworks as efficient adsorbents for high and selective removal of an aryl-organophosphorus flame retardant from water. ACS Appl Mater Interfaces 10:30265–30272
Simonin JP (2016) On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem Eng J 300:254–263
Li LJ, Liu FQ, Jing XS, Ling PP, Li AM (2011) Displacement mechanism of binary competitive adsorption for aqueous divalent metal ions onto a novel IDA-chelating resin: isotherm and kinetic modeling. Water Res 45:1177–1188
Wu H, Kim SY, Miwa M, Matsuyama S (2021) Synergistic adsorption behavior of a silica-based adsorbent toward palladium, molybdenum, and zirconium from simulated high-level liquid waste. J Hazard Mater 5:125136
Yi QP, Fan RY, Xie F, Zhang QL, Zhengrong L (2016) Recovery of Palladium(II) from nitric acid medium using a natural resin prepared from persimmon dropped fruits residues. J Taiwan Inst Chem Eng 61:299–305
Mu WJ, Du SZ, Li XL, Yu QH, Wei HY, Yang YC, Peng SM (2019) Removal of radioactive palladium based on novel 2D titanium carbides. Chem Eng J 358:283–290
Zha MQ, Liu J, Wong YL, Xu ZT (2015) Extraction of palladium from nuclear waste-like acidic solutions by a metal–organic framework with sulfur and alkene functions. J Mater Chem A 3:3928–3934
Ruhela R, Singh KK, Tomar BS, Sharma JN, Kumar M, Hubli RC, Suri AK (2012) Amberlite XAD-16 functionalized with 2-acetyl pyridine group for the solid phase extraction and recovery of palladium from high level waste solution. Sep Purif Technol 99:36–43
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H., Kudo, T., Takahashi, T. et al. Impregnation of covalent organic framework into porous silica support for the recovery of palladium ions from simulated high-level liquid waste. J Radioanal Nucl Chem 330, 1065–1074 (2021). https://doi.org/10.1007/s10967-021-07971-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07971-x