Abstract
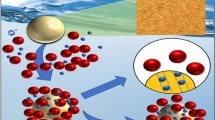
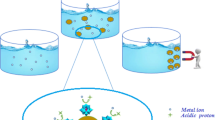
The adsorption of molybdenum (Mo) ions on ionic liquid-loaded XAD-7 resin containing 1-butyl-3-methylimidazolium(2,4,6-trimethyl)benzodithioate from acidic feed solutions was investigated. The novel loaded resin could adsorb more than 99.0% Mo ions from nitric acid solutions at a concentration between 0.050 and 0.50 M; however, the adsorption percentage of U(VI), Cs(I), Ce(III), Zr(IV) and Ru(III) ions was very low. With the further increase in the concentration of nitric acid, the distribution coefficient (Kd) of Mo ions by the loaded resin decreased. The separation factor (SFMo/U) was about 5000 at 0.50 M nitric acid. X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, and Raman spectroscopy results showed that Mo coordinated with sulfur, and part of Mo(VI) was reduced to Mo(IV). The results of column experiment indicated that about 94.7% Mo ions with a purity of over 99.0% was obtained by eluting with different concentrations of nitric acid.
Graphic abstract











Similar content being viewed by others
References
OECD-NEA (2017) The Supply of Medical Radioisotopes 2017 medical isotope supply review: 99Mo/99mTc market demand and production capacity projection
Lee S-K, Beyer GJ, Lee JS (2016) Development of industrial-scale fission 99Mo production process using low enriched Uranium target. Nucl Eng Technol 48:613–623
McDonald MJ, Carson SD, Naranjo GE, Wemple JA (2000) Challenges of extracting and purifying fission-produced molybdenum-99. Ind Eng Chem Res 39:3146–3150
Tkac P, Paulenova A (2008) Speciation of molybdenum (VI) in aqueous and organic phases of selected extraction systems. Sep Sci Technol 43:2641–2657
Mandal S, Mandal A, Lahiri S (2013) Species dependent extraction of 99Mo. J Radioanal Nucl Chem 295:861–863
Zeng L, Cheng CY (2009) A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts Part II: separation and purification. Hydrometallurgy 98:10–20
Xia CT, Fuenzalida VM (2003) Room temperature electrochemical growth of polycrystalline BaMoO4 films. J Eur Ceram Soc 23:519–525
Wu J, Wei C, Li X, Wang S, Wang M, Li C (2012) Selective extraction of Mo using Cyanex-272 and tributyl phosphate from low grade Ni-Mo ore leach liquor. Sep and Purif Technol 99:120–126
Xiao C, Zeng L, Xiao L, Zhang G (2017) Solvent extraction of molybdenum(VI) from hydrochloric acid leach solutions using P507. Part I: Extraction Mech Solvent Extr Ion Exch 35:130–144
Saberyan K, Maragheh MG, Ashtari P, Alamdari SK (2003) Liquid-liquid extraction of molybdenum(VI) from acidic media with Cyanex-301. Miner Eng 16:391–393
Saily A, Khurana U, Yadav SK, Tandon SN (1996) Thiophosphinic acids as selective extractants for molybdenum recovery from a low grade ore and spent catalysts. Hydrometallurgy 41:99–105
Lasheen TA, Ibrahim ME, Hassib HB, Helal AS (2014) Recovery of molybdenum from Uranium bearing solution by solvent extraction with 5-Nonylsalicylaldoxime. Hydrometallurgy 146:175–182
Barik SP, Park KH, Parhi PK, Kim DJ, Nam CW (2014) Separation and recovery of molybdenum from acidic solution using LIX 973N. Sep Sci Technol 49:647–655
Tkac P, Brown MA, Momen A, Wardle KE, Copple JM, Vandegrift GF (2018) MOEX: Solvent extraction approach for recycling enriched Mo98/Mo100 material. Sep Sci Technol 53:1856–1863
Sato T, Watanabe H, Suzuki H (1986) Liquid-liquid extraction of molybdenum(VI ) from aqueous acid-solutions by high-molecular-weight amines. Solvent Extr Ion Exch 4:987–998
Sahu KK, Agrawal A, Mishra D (2013) Hazardous waste to materials: Recovery of molybdenum and vanadium from acidic leach liquor of spent hydroprocessing catalyst using alamine 308. J Environ Manage 125:68–73
Leoncini A, Huskens J, Verboom W (2017) Ligands for f-element extraction used in the nuclear fuel cycle. Chem Soc Rev 46:7229–7273
Veliscek-Carolan J (2016) Separation of actinides from spent nuclear fuel: a review. J Hazard Mater 318:266–281
Sun X, Luo H, Dai S (2012) Ionic liquids-based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem Rev 112:2100–2128
Dong K, Liu X, Dong H, Zhang X, Zhang S (2017) Multiscale studies on ionic liquids. Chem Rev 117:6636–6695
Jones MB, Gaunt AJ (2013) Recent developments in synthesis and structural chemistry of nonaqueous actinide complexes. Chem Rev 113:1137–1198
Villa R, Alvarez E, Porcar R, Garcia-Verdugo E, Luis SV, Lozano P (2019) Ionic liquids as an enabling tool to integrate reaction and separation processes. Green Chem 21:6527–6544
Quijada-Maldonado E, Torres MJ, Romero J (2017) Solvent extraction of molybdenum (VI) from aqueous solution using ionic liquids as diluents. Sep Purif Technol 177:200–206
Quijada-Maldonado E, Sanchez F, Perez B, Tapia R, Romero J (2018) Task-specific ionic liquids as extractants for the solvent extraction of molybdenum(VI) from aqueous solution using different commercial ionic liquids as diluents. Ind Eng Chem Res 57:1621–1629
Vandegrift GF, Snelgrove JL, Aase S (1999) Converting targets and processes for fission-product molybdenum-99 from high- to low-enriched uranium. IAEA
Bernhard G (1994) Separation of radionuclides from a fission-production by ion-exchange on alumina. J Radioanal Nucl Chem 177:321–325
van der Walt TN, Coetzee PP (2004) The isolation of Mo-99 from fission material for use in the Mo-99/Tc-99m generator for medical use. Radiochim Acta 92:251–257
Rao A, Sharma AK, Kumar P, Charyulu MM, Tomar BS, Ramakumar KL (2014) Studies on separation and purification of fission Mo-99 from neutron activated uranium aluminum alloy. Appl Radiat Isot 89:186–191
Ansari SA, Mohapatra PK (2017) A review on solid phase extraction of actinides and lanthanides with amide based extractants. J Chromatogr A 1499:1–20
Zhang A, Zhang W, Wang Y, Ding X (2016) Effective separation of cesium with a new silica-calix 4 biscrown material by extraction chromatography. Sep Purif Technol 171:17–25
Gujar RB, Ansari SA, Verboom W, Mohapatra PK (2018) Highly efficient extraction chromatography resins containing dendrimers with DGA groups in ionic liquid for actinide uptake. Ind Eng Chem Res 57:13226–13234
Van de Voorde M, Van Hecke K, Binnemans K, Cardinaels T (2020) Supported ionic liquid phases for the separation of samarium and europium in nitrate media: Towards purification of medical samarium-153. Sep Purif Technol 232:115939
Mahanty B, Mohapatra PK (2020) Highly efficient separation of thorium from uranium in nitric acid feeds by solid phase extraction using Aliquat 336. Sep Purif Technol 237:116318
Hawkins CA, Momen MA, Garvey SL, Kestell J, Kaminski MD, Dietz ML (2015) Evaluation of solid-supported room-temperature ionic liquids containing crown ethers as media for metal ion separation and preconcentration. Talanta 135:115–123
Falaras P, Mitsopoulou CA, Argyropoulos D, Lyris E, Psaroudakis N, Vrachnou E, Katakis D (1995) Synthesis, cyclic voltammetric eletrospray mass-spectrometric studies of a series of tris-substituted 1,2-dithiolene complexes of tungsten and molybdenum. Inorg Chem 34:4536–4542
Fomitchev DV, Lim BS, Holm RH (2001) Electron distribution in the nonclassical bis(dithiolene) electron transfer series M(CO)2(S2C2Me2)20/1-/2- (M = Mo, W): Assessment by structural, spectroscopic, and density functional theory results. Inorg Chem 40:645–654
Yang XG, Freeman GKW, Rauchfuss TB, Wilson SR (1991) The dithiocarbonate route to 1,2-dithiolene complexes of molybdenum and tungsten. Inorg Chem 30:3034–3038
Tian ZQ, Donahue JP, Holm RH (1995) Synthesis of new types of dithiolene ligands. Inorg Chem 34:5567–5572
Grote J, Friedrich F, Berthold K, Hericks L, Neumann B, Stammler H-G, Mitzel NW (2018) Dithiocarboxylic acids: an old theme revisited and augmented by new preparative, spectroscopic and structural facts. Chem Eur J 24:2626–2633
Bonhote P, Dias AP, Papageorgiou N, Kalyanasundaram K, Gratzel M (1996) Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem 35:1168–1178
Hwang DS, Choung WM, Kim YK, Park JH, Park SJ (2002) Separation of 99Mo from a simulated fission product solution by precipitation with a-benzoinoxime. J Radioanal Nucl Chem 254:255–262
Horwitz EP, Chiarizia R, Dietz ML (1992) A novel stronitum-selective extraction chromatographic resin. Solvent Extr Ion Exch 10:313–336
Gebreyohannes AY, Upadhyaya L, Silva LP, Falca G, Carvalho PJ, Nunes SP (2020) Hollow fibers with encapsulated green amino acid-based ionic liquids for dehydration. ACS Sustain Chem Eng 8:17763–17771
Bell TJ, Ikeda Y (2011) The application of novel hydrophobic ionic liquids to the extraction of uranium(VI) from nitric acid medium and a determination of the uranyl complexes formed. Dalton Trans 40:10125–10130
Dadachova K, La Riviere K, Anderson P (1999) Improved processes of molybdenum-99 production. J Radioanal Nucl Chem 240:935–938
Sasaki T, Kobayashi T, Takagi I, Moriyama H (2008) Hydrolysis constant and coordination geometry of zirconium(IV). J Nucl Sci Technol 45:735–739
Saptiama I, Kaneti YV, Suzuki Y, Tsuchiya K, Fukumitsu N, Sakae T, Kim J, Kang Y-M, Ariga K, Yamauchi Y (2018) Template-free fabrication of mesoporous alumina nanospheres using post-synthesis water-ethanol treatment of monodispersed aluminium glycerate nanospheres for molybdenum adsorption, Small 14:1800474
Kondekar NP, Boebinger MG, Woods EV, McDowell MT (2017) In Situ XPS Investigation of transformations at crystallographically oriented MoS2 interfaces. ACS Appl Mater Interf 9:32394–32404
Lince JR, Pluntze AM, Jackson SA, Radhakrishnan G, Adams PM (2014) Tribochemistry of MoS3 nanoparticle coatings. Tribol Lett 53:543–554
de Barros’Bouchet MIMI, Martin JMJM, Le-Mogne TT, Vacher BB (2005) Boundary lubrication mechanisms of carbon coatings by MoDTC and ZDDP additives. Tribol Int 38:257–264
Xiao C, Zeng L, Xiao L, Zhang G (2017) Solvent extraction of molybdenum (VI) from hydrochloric acid leach solutions using P507 Part I: extraction and mechanism. Solvent Extr Ion Exc 35:130–144
Chen GJJ, McDonald JW, Bravard DC, Newton WE (1985) Synthetic utility of molybdenum diazene adducts-products, preparation, reactions, and spectral properties of oxo-free and (18O)oxo molybdenum complexes, lnorg. Chem 24:2327–2333
Singh R, Mahandra H, Gupta B (2018) Optimization of a solvent extraction route for the recovery of Mo from petroleum refinery spent catalyst using Cyphos IL 102. Solvent Extr Ion Exc 36:401–419
Houben J (1906) Carbi-thio-acids. I. Arylcarbi-thio-acids. Ber Dtsch Chem Ges 39:3219–3233
Kato S, Nishiwaki M, Inagaki S, Ohshima S, Ohno Y, Mizuta M, Murai T (1985) Preparation and characterization of bis(thioacyl) trisulfides and tetrasulfides. Chem Ber Recl 118:1684–1695
Tatsumisago M, Matsubayashi G, Tanaka T, Nishigaki S, Nakatsu K (1982) Synthesis, spectroscopy, and X-ray crystallographic analysis of (η3-dithiobenzoato-SCS')oxo(trithioperoxybenzoato-S,S'S'')molybdenum(IV) and μ-oxo-bis[bis(dithiobenzoato-SS')oxomolybdenum(V)], J Chem Soc, Dalton Trans. 121–127
Hlova IZ, Dolotko O, Boote BW, Pathak AK, Smith EA, Pecharsky VK, Balema VP (2018) Multi-principal element transition metal dichalcogenides via reactive fusion of 3D-heterostructures. Chem Commun 54:12574–12577
Wang Y, Zhou Y, Han M, Xi Y, You H, Hao X, Li Z, Zhou J, Song D, Wang D, Gao F (2019) Environmentally-friendly exfoliate and active site self-assembly: thin 2D/2D heterostructure amorphous nickel-iron alloy on 2D materials for efficient oxygen evolution reaction, Small 15:1805435
Yue Q, Wang L, Fan H, Zhao Y, Wei C, Pei C, Song Q, Huang X, Li H (2021) Wrapping plasmonic silver nanoparticles inside one-dimensional nanoscrolls of transition-metal dichalcogenides for enhanced photoresponse. Inorg Chem 60:4226–4235
Griffith WP, Nogueira HIS, Parkin BC, Sheppard RN, White AJP, Williams DJ (1995) Second-row and third-row transition-metal complex of dihydroxybenzonic acids, and the crystal-structure of [NME4]2[MoO2(2,3-DHB)2]·1.5H2O (2,3-H2DHB=2,3-dihyroxybenzoc acid), J Chem Soc, Dalton Trans, 1775–1781
Whiting GT, Bartley JK, Dummer NF, Hutchings GJ, Taylor SH (2014) Vanadium promoted molybdenum phosphate catalysts for the vapour phase partial oxidation of methanol to formaldehyde. Appl Catala A-Gen 485:51–57
Morgan SH, Magruder RH (1990) Raman-spectra of molybdenum phosphate-glasses and some crystalline analogs. J Am Ceram Soc 73:753–756
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. U1967216, 21976008, and 11575010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Z., Chu, T. Selective adsorption of molybdenum ions on ionic liquid-loaded resin containing 1-butyl-3-methylimidazolium(2,4,6-trimethyl)benzodithioate. J Radioanal Nucl Chem 330, 775–784 (2021). https://doi.org/10.1007/s10967-021-07970-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07970-y