Abstract
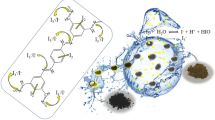
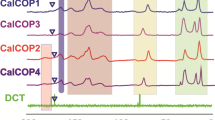
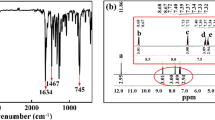
The radioactive iodine in nuclear waste is increasingly polluting the environment, and the careful handling of radioactive iodine is crucial to the sustainable development of nuclear energy. In this paper, a imine-based Covalent organic polymer (COP) is prepared by aldimine condensation. The COP material is stable under 500 MPa ultra-high pressure with an average particle size of 100 μm. The adsorption process is in line with the Langmuir isotherm adsorption model control, and the maximum adsorption capacity is 3082.14 mg/g. The adsorption is a spontaneous endothermic process of increased entropy and raising the temperature is conducive to the adsorption process. The electron donor and iodine electron acceptor in the ligand form an electron transfer compound to achieve iodine adsorption.










Similar content being viewed by others
References
Zhang X, da Silva I, Godfrey HGW, Callear SK, Sapchenko SA, Cheng Y, Vitórica-Yrezábal I, Frogley MD, Cinque G, Tang CC, Giacobbe C, Dejoie C, Rudić S, Ramirez-Cuesta AJ, Denecke MA, Yang S, Schröder M (2017) Confinement of Iodine Molecules into Triple-Helical Chains within Robust Metal-Organic Frameworks. J Am Chem Soc 139:16289–16296
Han T-T, Wang L-N, Potgieter JH (2020) ZIF-11 derived nanoporous carbons with ultrahigh uptakes for capture and reversible storage of volatile iodin. J Solid State Chem 282:121108
Jong-Ho P, Ralph TY (2005) Predicting adsorption isotherms of low-volatile compounds by temperature programmed desorption: iodine on carbon. Langmuir 21:5055–5060
Ruidan M, Fengyuan W, Junyu L, Huadong G, Ting Z, Shuang L, Zhiyong G, Xianmin G (2020) An amino-decorated porous metal-organic framework for efficient C2 hydrocarbon/CH4 separation and high iodine adsorption. Microporous Mesoporous Mater 305:110306
Hui L, Xuesong D, Bao-Hang H (2016) porous azo-bridged porphyrin–phthalocyanine network with high iodine capture capability. Chem-Eur J 22:1–7
Cheng PY, Zhong D, Zewail AH (1995) Microscopic solvation and femtochemistry of charge-transfer reactions: the problem of benzene(s)-iodine binary complexes and their solvent structures. Chem Phys Lett 242:369–379
Al-Hashimi NA (2004) Spectroscopic studies of the reaction of iodine with 2,3-diaminopyridine. Spectrochim Acta A 60:2181–2184
Al-Hashimi NA, Hassan KA, Nour E-M (2005) Electronic, infrared, and Raman spectral studies of the reaction of iodine with 4-aminopyridine. Spectrochim Acta A 62:317–321
Miyazaki T, Katayama S, Funai E, Tsuji Y, Sakurai S (2005) Role of adsorbed iodine into poly (vinyl alcohol) films drawn in KI/I2 solution. Polym J 46:7436–7442
Shuai Y, Liang F, Kecheng W, Jiandong P, Matheiu B, Christina L, Yujia S, Junsheng Q, Xinyu Y, Peng Z, Qi W, Lanfang Z, Yingmu Z, Liangliang Z, Yu F, Jialuo L, Hong-Cai Z (2018) Stable metal–organic frameworks: design, synthesis, and applications. Adv Mater 30:1704303
Kim S, Lim H, Lee J, Choi HC (2018) Synthesis of a scalable two-dimensional covalent organic framework (COF) by photon-assisted imine condensation reaction on the water surface. Langmuir 34:8731–8738
Wang L-M, Yue J-Y, Cao X-Y, Wang D (2019) Insight into the transimination process in the fabrication of surface schiff-based covalent organic frameworks. Langmuir 35:6333–6339
Elewa AM, Elsayed MH, EL-Mahdy AFM, Chang C-L, Ting L-Y, Lin W-C, Lu C-Y, Chou H-H (2021) Triptycene-based discontinuously-conjugated covalent organic polymer photocatalysts for visible-light-driven hydrogen evolution from water. Appl Catal B-Environ 285:119802
Xiang Z, Mercado R, Huck JM, Wang H, Guo Z, Wang W, Cao D, Haranczyk M, Smit B (2015) Systematic tuning and multifunctionalization of covalent organic polymers for enhanced carbon capture. S J Am Chem Soc 137:13301–13307
Banerjee A, Herrero S, Gutiérrez Á, Chattopadhyay S (2020) Synthesis, structure and magnetic property of a dinuclear cobalt (II/III) complex with a reduced Schiff base ligand. Polyhedron 190:114756
Vadivel T, Dhamodaran M (2016) Synthesis, characterization and antibacterial studies of ruthenium (III) complexes derived from chitosan Schiff base. Int J Biol Macromo 90:44–52
Jiang S-Y, Gan S-X, Zhang X, Li H, Qi Q-Y, Cui F-Z, Lu J, Zhao X (2019) Aminal-linked covalent organic frameworks through condensation of secondary amine with aldehyde. J Am Chem Soc 141:14981–14986
Wang X, Shi X, Wang Y (2020) In-situ growth of cationic covalent organic frameworks (COFs) for mixed matrix membranes with enhanced performances. Langmuir 36:10970–10978
Shan H, Li S, Yang Z, Zhang X, Zhuang Y, Zhu Q, Cai D, Qin P, Baeyens J (2021) Triazine-based N-rich porous covalent organic polymer for the effective detection and removal of Hg (II) from an aqueous solution. Chem Hem J 426:130757
Skorjanc T, Shetty D, Trabolsi A (2021) Pollutant removal with organic macrocycle-based covalent organic polymers and frameworks. Chem 7(4):882–918
Şenol ZM, Şimşek S, Özer A, Arslan DŞ (2020) Synthesis and characterization of chitosan–vermiculite composite beads for removal of uranyl ions: isotherm, kinetics and thermodynamics studies. J Radioanal Nucl Ch 327:159–173
Şenol ZM, Gül ÜD, Şimşek S (2019) Assessment of Pb 2+ removal capacity of lichen (Evernia prunastri): application of adsorption kinetic, isotherm models, and thermodynamics. Environ Sci Pollut R 26(26):27002–27013
Fan H, Mundstock A, Feldhoff A, Knebel A, Gu J, Meng H, Caro J (2018) COF–COF bilayer membranes for highly selective gas separation. J Am Chem Soc 140:1–6
Maza S, Kijatkin C, Bouhidel Z, Pillet S, Schaniel D, Imlau M, Guillot B, Cherouana A, Bendeif E-E (2020) Synthesis, structural investigation and NLO properties of three 1,2,4-triazole Schiff bases. J Mol Struct 1219:128492
Xu H-S, Luo Y, Li X, See PZ, Chen Z, Ma T, Liang L, Leng K, Abdelwahab I, Wang L, Li R, Shi X, Zhou Y, Lu XF, Zhao X, Liu C, Sun J, Loh KP (2020) Single crystal of a one-dimensional metallo-covalent organic framework. Nat Commun 11:1–2
Sun Y, Gao H, Xu L, Waterhouse GIN, Zhang H, Qiao X, Xu Z (2020) Ultrasensitive determination of sulfathiazole using a molecularly imprinted electrochemical sensor with CuS microflowers as an electron transfer probe and Au@COF for signal amplification. Food Chem 332:127376
Zhongping L, Xiao F, Yongcun Z, Yuwei Z, Hong X, Xiaoming L, Ying M (2014) A 2D azine-linked covalent organic framework for gas storage applications. Chem Commun (Camb) 50:13825–13828
Refat MS, El-Sayed MY, Adam AMA (2013) Cu(II), Co(II) and Ni(II) complexes of new Schiff base ligand: Synthesis, thermal and spectroscopic characterizations. J Mol Struct 1038:62–72
Wang Y, Chen Y, Liu C, Yu F, Chi Y (2017) The effect of magnesium oxide morphology on adsorption of U(VI) from aqueous solution. Chem Eng J 316:936–950
Enol ZM (2020) Effective biosorption of allura red dye from aqueous solutions by the dried-lichen (pseudoevernia furfuracea) biomass. Int J Environ 1–15
Drweesh SA, Fathy NA, Wahba MA, Hanna AA, Akarish AIM, Elzahany EAM, El-Sherif IY, Abou-El-Sherbini KS (2016) Equilibrium, kinetic and thermodynamic studies of Pb(II) adsorption from aqueous solutions on HCl-treated Egyptian kaolin. J Environ Chem Eng 4(2):1674–1684
Şenol ZM, Gul UD, Gurkan R (2020) Bio-sorption of bisphenol a by the dried- and inactivated-lichen (Pseudoevernia furfuracea) biomass from aqueous solutions. J Environ Health Sci 18(2):853–864
Daneshvar E, Kousha M, Jokar M, Koutahzadeh N, Guibal E (2012) Acidic dye biosorption onto marine brown macroalgae: Isotherms, kinetic and thermodynamic studies. Chem Eng J 204–206:225–234
Hameed BH, Tan IAW, Ahmad AL (2008) Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on co-conut husk-based activated carbon. Chem Eng J 144(1):235–244
Li AY, Deng H, Jiang YH, Ye CH, Yu BG, Zhou XL, Ma AY (2020) Superefficient removal of heavy metals from wastewater by Mg-loaded biochars: adsorption characteristics and removal mechanisms. Langmuir 36:9160–9174
Gatabi MP, Moghaddam HM, Ghorbani M (2016) Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J Mol Liq 216:117–125
Şimşek S, Şenol ZM, Ulusoy Hİ (2017) Synthesis and characterization of a composite polymeric material including chelating agent for adsorption of uranyl ions. J Hazard Mater 338:437–446
Kavak DD, Uelkue S (2015) Kinetic and equilibrium studies of adsorption of β-glucuronidase by clinoptilolite-rich minerals. Process Biochem 50:221–229
Yu F, Chen Y, Wang Y, Liu C, Ma W (2018) Enhanced removal of iodide from aqueous solution by ozonation and subsequent adsorption on Ag-Ag2O modified on Carbon Spheres. Appl Surf Sci 427:753–762
Li Z-J, Yue Z, Ju Y, Wu X, Ren Y, Wang S, Li Y, Zhang Z-H, Guo X, Lin J, Wang J-Q (2020) Ultrastable thorium metal–organic frameworks for efficient iodine adsorption. Inorg Chem 59:4435–4442
Tang PH, So PB, Lee KR, Lai YL, Lee CS, Lin CH (2020) Metal organic framework-polyethersulfone composite membrane for iodine capture. Polym J 12:2309–2309
Li Z-J, Ju Y, Lu H, Wu X, Yu X, Li Y, Wu X, Zhang Z-H, Lin J, Qian Y, He M-Y, Wang J-Q (2021) Boosting the iodine adsorption and radioresistance of Th-UiO-66 MOFs via aromatic substitution. Chem Eur J 4:1162–1162
Ye F, Huang C, Jiang X, He W, Gao X, Ma L, Ao J, Xu L, Wang Z, Li Q, Li J, Ma H (2020) Reusable fibrous adsorbent prepared via Co-radiation induced graft polymerization for iodine adsorption. Ecotoxicol Environ Saf 203:111021
Liu R, Zhang W, Chen Y, Xu C, Hu G, Han Z (2020) Highly efficient adsorption of iodine under ultrahigh pressure from aqueous solution. Sep Purif Technol 233:115999
Geng T, Chen G, Xia H, Zhang W, Zhu Z, Cheng B (2018) Poly{tris[4-(2-thienyl) phenyl] amine} and poly [tris (4-carbazoyl- 9-yl phenyl) amine] conjugated microporous polymers as absorbents for highly efficient iodine adsorption. J Solid State Chem 26:85–91
Chen P, He X, Pang M, Dong X, Zhao S, Zhang W (2020) Iodine capture using zr-based metal-organic frameworks (Zr-MOFs): adsorption performance and mechanism. ACS Appl Mater Inter 12:20429–20439
Li G, Huang Y, Lin J, Yu C, Liu Z, Fang Y, Xue Y, Tang C (2020) Effective capture and reversible storage of iodine using foam-like adsorbents consisting of porous boron nitride microfibers. Chem Eng J 382:122833
Long X, Chen Y-S, Zheng Q, Xie X-X, Tang H, Jiang L-P, Jiang J-T, Qiu J-H (2020) Removal of iodine from aqueous solution by PVDF/ZIF-8 nanocomposite membranes. Sep Purif Technol 238:116488
Li Z-J, Ju Y, Yu B, Wu X, Lu H, Li Y, Zhou J, Guo X, Zhang Z-H, Lin J, Wang J-Q, Wang S (2020) Modulated synthesis and isoreticular expansion of Th-MOFs with record high pore volume and surface area for iodine adsorption. Chem Commun (Camb) 56:6715–6718
Acknowledgements
Thanks for the support of the National Natural Science Foundation of China (No.21667024).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conceptualization and methodology. YW: Investigation, Data Curation, Writing- Original draft preparation, Visualization. YC: Resources, Supervision, Writing-Reviewing and Editing, Project Administration. MZ: Software, Validation; LZ: Investigation.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhao, M., Zhang, L. et al. Covalent organic polymers are highly effective absorbers of iodine in water under ultra-high pressure. J Radioanal Nucl Chem 329, 1407–1415 (2021). https://doi.org/10.1007/s10967-021-07900-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07900-y