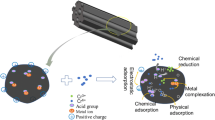
Abstract
Pyrolytic biochar and hydrothermal biochar of corn stalk were prepared at different temperatures, and their abilities to remove iodate were compared. The characterization results show that the preparation temperature determines the degree of carbonization and the specific surface area of biochar. Pyrolytic biochar has a larger specific surface area than hydrothermal biochar, but retains fewer oxygen-containing functional groups. The adsorption capacity of hydrothermal biochar (16.87 mg g−1) is better than that of pyrolytic biochar (10.31 mg g−1). Corn stalk hydrothermal biochar is fully recyclable for multiple adsorption/desorption trials, which makes it extremely attractive for iodate contaminant separation.
Graphic abstract










Similar content being viewed by others
References
Balter M (1995) Radiation biology: Chernobyl’s thyroid cancer toll. Science 270:1758–1759
Xu S, Zhang L, Freeman SPHT, Hou X, Shibata Y, Sanderson D, Cresswell A, Doi T, Tanaka A (2015) Speciation of radiocesium and radioiodine in aerosols from tsukuba after the fukushima nuclear accident. Environ Sci Technol 49(2):1017–1024. https://doi.org/10.1021/es504431w.406
Moore RC, Pearce CI, Morad JW, Chatterjee S, Levitskaia TG, Asmussen RM, Lawter AR, Neeway JJ, Qafoku NP, Rigali MJ, Saslow SA, Szecsody JE, Thallapally PK, Wang G, Freedman VL (2019) Iodine immobilization by materials through sorption and redox-driven processes: a literature review. Sci Total Environ S0048–9697(19):32737–32738. https://doi.org/10.1016/j.scitotenv.2019.06.166
Lin J, Liu Y, Zhang GH (2015) Research progress in the removal of radioactive iodonucleoid from the water body. Ind Water Treat 35:10–18
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interfac 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Elaigwu SE, Rocher V, Kyriakou G, Greenway GM (2014) Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell. J Ind Eng Chem 20(5):3467–3473. https://doi.org/10.1016/j.jiec.2013.12.036
Tong Y, McNamara PJ, Mayer BK (2019) Adsorption of organic micropollutants onto biochar: a review of relevant kinetics, mechanisms and equilibrium. Environ Sci: Water Res Technol 5(5):821–838. https://doi.org/10.1039/c8ew00938d
Han X, Chu L, Liu S, Chen T, Ding C, Yan J, Quan G (2015) Removal of methylene blue from aqueous solution using porous biochar obtained by KOH activation of peanut shell biochar. BioResources 10(2):2836–2849. https://doi.org/10.15376/biores.10.2.2836-2849
Zhang P, Li Y, Cao Y, Han L (2019) Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour Technol 285:121348. https://doi.org/10.1016/j.biortech.2019.121348
Chen T, Zhang N, Xu Z, Hu X, Ding Z (2018) Integrated comparisons of thorium(IV) adsorption onto alkali-treated duckweed biomass and duckweed-derived hydrothermal and pyrolytic biochar. Environ Sci Pollut R 26(3):2523–2530. https://doi.org/10.1007/s11356-018-3789-x
Hansen V, Müller-Stöver D, Ahrenfeldt J, Holm JK, Henriksen UB, Hauggaard-Nielsen H (2015) Gasification biochar as a valuable by-product for carbon sequestration and soil amendment. Biomass Bioenerg 72:300–308. https://doi.org/10.1016/j.biombioe.2014.10.013
Meng J, Wang L, Liu X, Wu J, Brookes PC, Xu J (2013) Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresource Technol 142:641–646. https://doi.org/10.1016/j.biortech.2013.05.086
Zhang K, Chen T (2018) Dried powder of corn stalk as a potential biosorbent for the removal of iodate from aqueous solution. J Environ Radioactiv 190:73–80. https://doi.org/10.1016/j.jenvrad.2018.05.008
Liu P, Chen T, Zheng J (2020) Removal of iodate from aqueous solution using diatomite/nano titanium dioxide composite as adsorbent. J Radioanal Nucl Chem 324(3):1179–1188. https://doi.org/10.1007/s10967-020-07161-1
Olgun A, Atar N (2012) Equilibrium, thermodynamic and kinetic studies for the adsorption of lead (II) and nickel (II) onto clay mixture containing boron impurity. J Ind Eng Chem 18(5):1751–1757
Ma F, Zhao B, Diao J (2016) Adsorption of cadmium by biochar produced from pyrolysis of corn stalk in aqueous solution. Water Sci Technol 74(6):1335–1345. https://doi.org/10.2166/wst.2016.319
Kumar P, Prajapati AK, Dixit S, Yadav VL (2020) Adsorption of fluoride from aqueous solution using biochar prepared from waste peanut hull. Mater Res Express 6(12):125553. https://doi.org/10.1088/2053-1591/ab6ca0
Mohan D, Sharma R, Singh VK, Steele P, Pittman CU (2012) Fluoride removal from water using bio-char, a green waste, low-cost adsorbent: equilibrium uptake and sorption dynamics modeling. Ind Eng Chem Res 51(2):900–914. https://doi.org/10.1021/ie202189v
Mehmood A, Bano S, Fahim A, Parveen R, Khurshid S (2015) Efficient removal of crystal violet and eosin B from aqueous solution using Syzygium cumini leaves: a comparative study of acidic and basic dyes on a single adsorbent. Korean J Chem Eng 32:882–895. https://doi.org/10.1007/s11814-014-0308-8
Liu L, Huang Y, Zhang S, Gong Y, Su Y, Cao J, Hu H (2019) Adsorption characteristics and mechanism of Pb(II) by agricultural waste-derived biochars produced from a pilot-scale pyrolysis system. Waste Manage 100:287–295. https://doi.org/10.1016/j.wasman.2019.08.021
Hamed MM (2014) Sorbent extraction behavior of a nonionic surfactant, triton X-100, onto commercial charcoal from low level radioactive waste. J Radioanal Nucl Chem 302(1):303–313. https://doi.org/10.1007/s10967-014-3250-7
Kuncham K, Nair S, Duran S, Bose R (2017) Efficient removal of uranium(VI) from aqueous medium using ceria nanocrystals: an adsorption behavioural study. J Radioanal Nucl Chem 313(1):101–112. https://doi.org/10.1007/s10967-017-5279-x
El-Maghrabi HH, Younes AA, Salem AR, Rabie K, El-shereafy E (2019) Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): preparation, characterization and adsorption optimization. J Hazard Mater 378:120703
Lei H, Zhou D, Tang J, Hu X, Pan N, Zou H, Chi F, Wang X (2020) Epoxy graphene oxide from a simple photo-Fenton reaction and its hybrid with phytic acid for enhancing U(VI) capture. Sci Total Environ 738:140316
Guo X, Yang H, Liu Q, Liu J, Chen R, Zhang H, Yu J, Zhang M, Li R, Wang J (2020) A chitosan-graphene oxide/ZIF foam with anti-biofouling ability for uranium recovery from seawater. Chem Eng J 382:122850
Ho Y-S (2004) Selection of optimum sorption isotherm. Carbon 42(10):2115–2116. https://doi.org/10.1016/j.carbon.2004.03.019
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10. https://doi.org/10.1016/j.cej.2009.09.013
Kılıç M, Kırbıyık Ç, Çepelioğullar Ö, Pütün AE (2013) Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl Surf Sci 283:856–862. https://doi.org/10.1016/j.apsusc.2013.07.033
Chang R (2000) Physical chemistry for the chemical and biological sciences, 3rd edn. University Science Books, USA
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434. https://doi.org/10.1016/j.molliq.2018.10.048
Chen T, Da T, Ma Y (2021) Reasonable calculation of the thermodynamic parameters from adsorption equilibrium constant. J Mol Liq 322:114980
Hamed MM, Rizk HE, Ahmed IM (2018) Adsorption behavior of zirconium and molybdenum from nitric acid medium using low-cost adsorbent. J Mol Liq 249:361–370. https://doi.org/10.1016/j.molliq.2017.11.049
Zhai M, Guo L, Zhang Y, Dong P, Qi G, Huang Y (2016) Kinetic parameters of biomass pyrolysis by TGA. BioResources 11(4):8548–8557
Uzun BB, Sarioğlu N (2009) Rapid and catalytic pyrolysis of corn stalks. Fuel Process Technol 90:705–716
Zhao J, Yu L, Ma H, Zhou F, Yang K, Wu G (2020) Corn stalk-based activated carbon synthesized by a novel activation method for high-performance adsorption of hexavalent chromium in aqueous solutions. J Colloid Interface Sci 578:650–659
Sevilla M, Maciá-Agulló JA, Fuertes AB (2011) Hydrothermal carbonization of biomass as a route for the sequestration of CO2: chemical and structural properties of the carbonized products. Biomass Bioenerg 35(7):3152–3159. https://doi.org/10.1016/j.biombioe.2011.04.032
Guo S, Dong X, Wu T, Shi F, Zhu C (2015) Characteristic evolution of hydrochar from hydrothermal carbonization of corn stalk. J Anal Appl Pyrolysis 116:1–9
Guiotoku M, Rambo CR, Hansel FA, Magalhães WLE, Hotza D (2009) Microwave-assisted hydrothermal carbonization of lignocellulosic materials. Mater Lett 63(30):2707–2709. https://doi.org/10.1016/j.matlet.2009.09.049
Lee JW, Kidder M, Evans BR, Paik S, Buchanan AC III, Garten CT, Brown RC (2010) Characterization of biochars produced from cornstovers for soil amendment. Environ Sci Technol 44(20):7970–7974. https://doi.org/10.1021/es101337x
Jiang T-Y, Jiang J, Xu R-K, Li Z (2012) Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 89(3):249–256. https://doi.org/10.1016/j.chemosphere.2012.04.028
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technol 102(3):3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018
Ahmed MB, Zhou JL, Ngo HH, Guo W (2016) Insight into biochar properties and its cost analysis. Biomass Bioenerg 84:76–86. https://doi.org/10.1016/j.biombioe.2015.11.002
Bogusz A, Oleszczuk P, Dobrowolski R (2015) Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour Technol 196:540–549
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428
Fan S, Sun Y, Yang T, Chen Y, Yan B, Li R, Chen G (2020) Biochar derived from corn stalk and polyethylene co-pyrolysis: characterization and Pb(II) removal potential. RSC Adv 10(11):6362–6376
Ahmad M, Lee SS, Dou X, Mohan D, Sung J-K, Yang JE, Ok YS (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technol 118:536–544. https://doi.org/10.1016/j.biortech.2012.05.042
Kim W-K, Shim T, Kim Y-S, Hyun S, Ryu C, Park Y-K, Jung J (2013) Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresource Technol 138:266–270. https://doi.org/10.1016/j.biortech.2013.03.186
Guo X, Zhang S, Shan XQ (2008) Adsorption of metal ions on lignin. J Hazard Mater 151:134–142
Ding ZH, Wu HL, Hu X (2017) Multiple characterization for mechanistic insights of Pb(II) sorption onto biochars derived from herbaceous plant, biosolid, and livestock waste. BioResources 12:6763–6772
Zhang K, Chen T (2018) Sorption and removal of iodate from aqueous solution using dried duckweed (Landoltia punctata) powder. J Environ Radioactiv 316(2):543–551. https://doi.org/10.1007/s10967-018-5807-3
Da T, Chen T (2020) Optimization of experimental factors on iodate adsorption: a case study of pomelo peel. J Radioanal Nucl Chem 326(1):511–526. https://doi.org/10.1007/s10967-020-07312-4
Zou Y, Chen T, Yuan G, Zhang K (2018) Sorption of iodine on Beishan granite: effect of speciation and humic acid. J Radioanal Nucl Chem 317(2):723–730. https://doi.org/10.1007/s10967-018-5945-7
Yu WB, Xu HF, Tan DY, Fang YH, Roden EE, Wan Q (2020) Adsorption of iodate on nanosized tubular halloysite. Appl Clay Sci. https://doi.org/10.1016/j.clay.2019.105407
Liu P, Chen T, Zheng J (2020) Removal of iodate from aqueous solution using diatomite/nano titanium dioxide composite as adsorbent. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-020-07161-1
Dai JL, Zhang M, Zhu YG (2004) Adsorption and desorption of iodine by various Chinese soils. Environ Int 30(4):525–530. https://doi.org/10.1016/j.envint.2003.10.007
Dai JL, Zhang M, Hu QH, Huang YZ, Wang RQ, Zhu YG (2009) Adsorption and desorption of iodine by various Chinese soils: II iodide and iodate. Geoderma 153(1–2):130–135. https://doi.org/10.1016/j.geoderma.2009.07.020
Tokunaga K, Takahashi Y, Tanaka K, Kozai N (2021) Effective removal of iodate by coprecipitation with barite: behavior and mechanism. Chemosphere 266:129104. https://doi.org/10.1016/j.chemosphere.2020.129104
Li D, Kaplan DI, Sams A, Powell BA, Knox AS (2018) Removal capacity and chemical speciation of groundwater iodide (I-) and iodate (IO3-) sequestered by organoclays and granular activated carbon. J Environ Radioact 192:505–512. https://doi.org/10.1016/j.jenvrad.2018.08.008
Szczepaniak W, Kościelna H (2002) Specific adsorption of halogen anions on hydrous γ-Al2O3. Anal Chim Acta 470(2):263–276. https://doi.org/10.1016/s0003-2670(02)00661-x
Sangsoo H, Wooyong U, Won-Seok K (2019) Development of bismuth-functionalized graphene oxide to remove radioactive iodine. Dalton Trans 48(2):478–485. https://doi.org/10.1039/c8dt03745k
Li D, Kaplan DI, Price KA (2019) Iodine immobilization by silver-impregnated granular activated carbon in cementitious systems. J Environ Radioact 208–209:106017
Wang GH, Qafoku NP, Szecsody JE, Strickland CE, Brown CF, Freedman VL (2019) Time-dependent iodate and iodide adsorption to Fe oxides. ACS Earth Space Chem 3(11):2415–2420. https://doi.org/10.1021/acsearthspacechem.9b00145
Acknowledgements
This work was financially supported by the Major National Science and Technology Specific Project of Large Advanced Pressurized Water Reactor Nuclear Power Plant (2019ZX06004009), National Natural Science Foundation of China (U1967212) and the Fundamental Research Funds for the Central Universities (2018ZD10).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Da, TX., Chen, T., He, WK. et al. Comprehensive comparisons of iodate adsorption onto corn stalk hydrothermal and pyrolytic biochar. J Radioanal Nucl Chem 329, 1277–1290 (2021). https://doi.org/10.1007/s10967-021-07874-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07874-x