Abstract
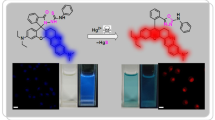
A new turn-off fluorescent sensor for trace analysis of uranyl ion (UO22+) in aqueous samples has been introduced in this study. Norfloxacin (1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1H quinoline-3-carboxylic acid) (NF) shows a strong fluorescence which was quenched at 446 nm in the presence of UO22+. Under optimized conditions, the present NF sensor has several noteworthy features, including sensitivity, wide linear range, lower detection limit, a broad pH range and fast response time. Besides that, the interaction properties and the fluorescent mechanism between the present NF sensor and UO22+ were investigated by density functional theory.











Similar content being viewed by others
References
Hill DJ (2008) Nuclear energy for the future. Nat Mater 7:680–682
Osman AA, Geipel G, Barkleit A, Bernhard G (2015) Uranium (VI) binding forms in selected human body fluids: thermodynamic calculations versus spectroscopic measurements. Chem Res Toxicol 28:238–247
Mathews G, Nagaiah N, Kumar MK, Ambika M (2015) Radiological and chemical toxicity due to ingestion of uranium through drinking water in the environment of Bangalore, India. J Radiol Prot 35:447–455
World Health Organization (2017) Guidelines for drinking water quality. Incorporating first addendum
Ruan C, Luo W, Wang W, Gu BH (2007) Surface-enhanced Raman spectroscopy for uranium detection and analysis in environmental samples. Anal Chim Acta 605:80–86
Jamali MR, Assadi Y, Shemirani F, Mohammad RMH, Reyhaneh RK, Majid MF, Masoud SN (2006) Synthesis of salicylaldehyde-modified mesoporous silica and its application as a new sorbent for separation, preconcentration and determination of uranium by inductively coupled plasma atomic emission spectrometry. Anal Chim Acta 579:68–73
Chandrasekaran K, Karunasagar D, Arunachalam J (2011) Dispersive liquid–liquid micro extraction of uranium(VI) from groundwater and seawater samples and determination by inductively coupled plasma–optical emission spectrometry and flow injection–inductively coupled plasma mass spectrometry. Anal Methods 3:2140–2147
Gonzalez JJ, Oropeza D, Mao X, Russo RE (2008) Assessment of the precision and accuracy of Thorium (232Th) and Uranium (238U) measured by quadrupole based Inductively Coupled Plasma-mass Spectrometry using liquid nebulization, nanosecond and femtosecond laser ablation. J Anal At Spectrom 23:229–234
Shenoy NS, Verma A, Kumar SA, Pandey S, Kumar SD, Reddy AV (2012) A comparative analysis of uranium in potable waters using laser fluorimetry and ICPMS techniques. J Radioanal Nucl Chem 294:413–417
Möser C, Kautenburger R, Philipp BH (2012) Complexation of europium and uranium by humic acids analyzed by capillary electrophoresis-inductively coupled plasma mass spectrometry. Electrophoresis 33:1482–1487
Peled Y, Krent E, Tal N, Tobias H, Mandler D (2014) Electrochemical determination of low levels of uranyl by a vibrating gold microelectrode. Anal Chem 87:768–776
Pin C, Zalduegui JS (1997) Sequential separation of light rare-earth elements, thorium and uranium by miniaturized extraction chromatography: application to isotopic analyses of silicate rocks. Anal Chim Acta 339:79–89
Wu D, Sedgwick AC, Gunnlaugsson T, Akkaya EU, Yoon J, James TD (2017) Fluorescent chemosensors: the past, present and future. Chem Soc Rev 46:7105–7123
Wu ML, Liao LF, Zhao MM, Lin YW, Xiao XL, Nie CM (2012) Separation and determination of trace uranium using a double-receptor sandwich supramolecule method based on immobilized salophen and fluorescence labeled oligonucleotide. Anal Chim Acta 729:80–84
Devore MA, Kerns SA, Gorden AEV (2015) Characterization of quinoxolinol salen ligands as selective ligands for chemosensors for uranium. Eur J Inorg Chem 14:5708
Maji S, Viswanathan KS (2009) Sensitization of uranium fluorescence using 2,6-pyridinedicarboxylic acid: application for the determination of uraniumin the presence of lanthanides. J Lumin 129:1242–1248
Elabd AA, Attia MS (2015) A new thin film optical sensor for assessment of UO22+ based on the fluorescence quenching of trimetazidine doped in sol gel matrix. J Lumin 165:179–184
Elabd AA, Attia MS (2016) Spectroflourimetric assessment of UO22+ by the quenching of the fluorescence intensity of Clopidogrel embedded in PMMA matrix. J Lumin 169:313–318
Elabd AA, Elhefnawy OA (2016) An efficient and sensitive optical sensor based on furosemide as a new fluoroionophore for determination of uranyl ion. J Fluoresc 26:271–276
Chen XT, He LF, Wang Y, Liu B, Tang YP (2014) Trace analysis of uranyl ion (UO22+) in aqueous solution by fluorescence turn-on detection via aggregation induced emission enhancement effect. Anal Chim Acta 847:55–60
Wen J, Huang Z, Hu S, Li S, Li WY, Wang WL (2016) Aggregation-induced emission active tetraphenylethene-based sensor for uranyl ion detection. J Hazard Mater 318:363–370
Shu X, Wang Y, Zhang S, Huang L, Wang S, Hua D (2015) Determination of trace uranyl ion by thermo responsive porphyrin–terminated polymeric sensor. Talanta 131:198–204
Ma J, He W, Han X, Hua D (2017) Amidoximated fluorescent polymer based sensor for detection of trace uranyl ion in aqueous solution. Talanta 168:10–15
Wua X, Maob Y, Wanga D, Huanga Q, Yinb Q, Zhengb M, Hua Q, Wang H (2020) Designing a colorimetric sensor containing nitrogen and oxygen atoms for uranyl ions identification: chromatic mechanism, binding feature and onsite application. Sensors Actuators B Chem 307:127681
Pérez-Ruiz T, Martínez-Lozano C, Tomás V, Carpena J (1997) Determination of Norfloxacin in real samples by different spectrofluorimetric techniques. Analyst 7:621–730
Kumar S, Kumar G, Tripathi AK, Seena S, Koh J (2018) Enhanced fluorescence norfloxacin substituted naphthalimide derivatives: Molecular docking and antibacterial activity. J Mol Struct 1157:292–299
Kaur B, Kumar R, Chand S, Singh K, Malik AK (2019) Determination of norfloxacin in urine and pharmaceutical samples using terbium doped zinc sulphide nanomaterials-sensitized fluorescence method. Spectrochim Acta Part A Mol Biomol Spectrosc 214:261–268
Han Y, Wu X, Yang J, Sun S (2005) The fluorescence characteristic of the yttrium–norfloxacin system and its analytical application. J Pharm Biomed Anal 38:528–531
Yuguang L, Ye L, Yuehui Z, Libin Y, Xin J, Ying W, Xinghua W (2012) Research and application of fluorescence system on norfloxacin-terbium-phen-SDBS. J Rare Earths 30842
Vogel AI (1975) A text book of quantitative inorganic analysis, 3rd edn. Longmans, London
Yang CT, Han J, Gu M, Liu J, Li Y, Huang Z, Yu HZ, Hu S, Wang XL (2015) Fluorescent recognition of uranyl ions by a phosphorylated cyclic peptide. Chem Commun 51:11769–11772
Hu Q, Zhang W, Yin Q, Wang Y, Wang H (2021) A conjugated fluorescent polymer sensor with amidoxime and polyfluorene entities for effective detection of uranyl ion in real samples. Spectrochim Acta Part A Mol Biomol Spectrosc 244:118864
Lin N, Ren W, Hu J, Gao B, Yuan D, Wang X, Fu J (2019) A novel tetraphenylethene-based fluorescent sensor for uranyl ion detection with aggregation-induced emission character. Dyes Pigm 166:182–188
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors state no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elabd, A.A., Elhefnawy, O.A. Uranyl ion assessment based on the fluorescence quenching of Norfloxacin. J Radioanal Nucl Chem 329, 935–944 (2021). https://doi.org/10.1007/s10967-021-07831-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07831-8