Abstract
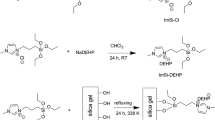
Quaternary ammonium salt functionalized silica gel (QA/SiO2) was synthesized and used to remove uranium from the alkaline solution. Batch experiments showed that the saturated adsorption capacity of QA/SiO2 to uranium was 370 mg/g at pH > 8.0. The isothermal adsorption confirms to the Langmuir model. Different co-existing anions have been studied, QA/SiO2 showed a high affinity for uranium. The interaction mechanism is anion exchange. Because of its low cost, operable large particle size and high adsorption capacity, it has a prospect of large-scale industrial application.







Similar content being viewed by others
References
Breeze P (2017) Nuclear Fuel and the Nuclear Resource. In: Nuclear Power. pp 11–20. https://doi.org/10.1016/b978-0-08-101043-3.00002-x
Herring JS (2018) Uranium and Thorium Resources. Nuclear Energy: A Volume in the Encyclopedia of Sustainability Science and Technology Series, second edition. doi:https://doi.org/10.1007/978-1-4939-6618-9_21
Humelnicu D, Dinu MV, Dragan ES (2011) Adsorption characteristics of UO(2)(2+) and Th(4+) ions from simulated radioactive solutions onto chitosan/clinoptilolite sorbents. J Hazard Mater 185(1):447–455. https://doi.org/10.1016/j.jhazmat.2010.09.053
Maksin DD, Nastasovic AB, Milutinovic-Nikolic AD, Surucic LT, Sandic ZP, Hercigonja RV, Onjia AE (2012) Equilibrium and kinetics study on hexavalent chromium adsorption onto diethylene triamine grafted glycidyl methacrylate based copolymers. J Hazard Mater 209–210:99–110. https://doi.org/10.1016/j.jhazmat.2011.12.079
Ding DX, Xin X, Li L, Hu N, Li GY, Wang YD, Fu PK (2014) Removal and recovery of U(VI) from low concentration radioactive wastewater by ethylenediamine-modified biomass of aspergillus niger. Water Air Soil Pollut 225(12):1–16. https://doi.org/10.1007/s11270-014-2206-4
Karayel Incili G, Aycık GA (2014) Chemical modification of silica gel with synthesized Schiff base hydrazone derivative and application for preconcentration and separation of U(VI) ions from aqueous solutions. J Radioanal Nucl Chem 301(2):417–426. https://doi.org/10.1007/s10967-014-3151-9
Tripathi S, Bose R, Roy A, Nair S, Ravishankar N (2015) Synthesis of hollow nanotubes of Zn2SiO4 or SiO2: mechanistic understanding and uranium adsorption behavior. ACS Appl Mater Interfaces 7(48):26430–26436. https://doi.org/10.1021/acsami.5b09805
Donia AM, Atia AA, Moussa EMM, El-Sherif AM, Abd El-Magied MO (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95(3–4):183–189. https://doi.org/10.1016/j.hydromet.2008.05.037
Shao L, Wang X, Ren Y, Wang S, Zhong J, Chu M, Tang H, Luo L, Xie D (2016) Facile fabrication of magnetic cucurbit[6]uril/graphene oxide composite and application for uranium removal. Chem Eng J 286:311–319. https://doi.org/10.1016/j.cej.2015.10.062
Elwakeel KZ, Atia AA, Guibal E (2014) Fast removal of uranium from aqueous solutions using tetraethylenepentamine modified magnetic chitosan resin. Bioresour Technol 160:107–114. https://doi.org/10.1016/j.biortech.2014.01.037
Cheira MF, Atia BM, Kouraim MN (2019) Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: Kinetic, equilibrium and thermodynamic studies. J Radiat Res Appl Sci 10(4):307–319. https://doi.org/10.1016/j.jrras.2017.07.005
Sreenivas T, Rajan KC (2013) Studies on the separation of dissolved uranium from alkaline carbonate leach slurries by resin-in-pulp process. Sep Purif Technol 112:54–60. https://doi.org/10.1016/j.seppur.2013.03.050
Yuan L-Y, Liao X-H, Liu Z-R, Chai Z-F, Shi W-Q (2017) U(VI) Extraction by 8-hydroxyquinoline: a comparison study in ionic liquid and in dichloromethane. Radiochim Acta 105(6):441–448. https://doi.org/10.1515/ract-2016-2664
Yinjie Song YW, Lianhe W, Chengxing S, ZhangZhun Y, Aimin Z (1999) Recovery of uranium from carbonate solutions using strongly basic anion exchanger 4 Column operation and quantitative analys. Eactive Funct Polym 39:245–252
Ladeira ACQ, Morais CA (2005) Uranium recovery from industrial effluent by ion exchange—column experiments. Miner Eng 18(13–14):1337–1340. https://doi.org/10.1016/j.mineng.2005.06.012
Ju YH, Sheng Dai OFW, Lin JS, Barnes CE (2000) Synthesis and characterization of ordered mesoporous anion-exchange inorganic/organic hybrid resins for radionuclide separation. Ind Eng Chem Res 39:550–553. https://doi.org/10.1021/ie990597v
Sayed MSE (2003) Uranium extraction from gattar sulfate leach liquor using aliquat-336 in a liquid emulsion membrane process. Hydrometallurgy, pp 51–56
Bholanath Mahanty PKM (2020) Highly efficient separation of Thorium from Uranium in nitric acid feeds by solid phase extraction using Aliquat 336. Sep Purif Technol 237:21. https://doi.org/10.1016/j.seppur.2019.116318
Ko YG, Lim J-M, Lee H, Chung KH, Kang MJ (2016) Sequential separation method for the determination of uranium and thorium in soil using diamyl amylphosphonate and Aliquat®336 impregnated polymer resins. React Funct Polym 106:43–50. https://doi.org/10.1016/j.reactfunctpolym.2016.07.007
Yinglin S, Nini C, Suliang Y, Xiaomin Li, Hong C, Guoxin T (2019) Quaternary phosphonium-grafted porous aromatic framework for preferential uranium adsorption in alkaline solution. Ind Eng Chem Res 58(39):18329–18335. https://doi.org/10.1021/acs.iecr.9b03580
Harris J, Rice SA (1988) A molecular dynamics study of the structure of a model Langmuir monolayer of amphiphile molecules. J Chem Phys 89(9):5898–5908. https://doi.org/10.1063/1.455541
Marczewski AW (2010) Analysis of kinetic Langmuir model Part I Integrated kinetic Langmuir equation (IKL) a new complete analytical solution of the Langmuir rate equation. Langmuir 26(19):15229–15238. https://doi.org/10.1021/la1010049
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant Nos. 21976075).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X.M., Dong, F.F., Huang, Y.W. et al. Synthesis of quaternary ammonium salt functionalized large-particle silica gel for removal of uranium. J Radioanal Nucl Chem 329, 171–177 (2021). https://doi.org/10.1007/s10967-021-07787-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07787-9