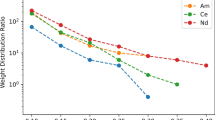
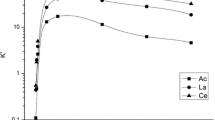
Abstract
In this work, the resin capacity factors of Ac, Am and Th on the DGA extraction chromatographic resin have been investigated between 0.5 and 2.0 M nitric acid without and with addition of elevated concentrations of the selective tetravalent complexant, 343HOPO. The resin capacity factors decreased with increasing 343HOPO concentration but remained high enough to support a selective removal of a tetravalent element. The Ac and Am capacity factors were also investigated under Th loading which yields generally reduced uptake on the resin. It was shown that loading effects could be fully scavenged by the addition of 343HOPO complexant. These results indicate that separation steps for Ac involving significant amounts of Th can be handled by selective tetravalent complexation with the DGA system.






Similar content being viewed by others
References
Deblonde J-P, Sturzbecher-Hoehne M, Abergel RJ (2013) Solution thermodynamic stability complexes formed with the octadentate hydroxypyridinonate ligand 3,4,3-LI(1,2-HOPO): a critical feature for efficient chelation of Lanthnide(IV) and Actinide(IV) ions. Inorg Chem 52:8805–8811
Deblonde J-P, Ricano A, Abergel RJ (2019) Ultra-selective ligand-driven separation of strategic actinides. Nat Commun 10:2438
Deblonde J-P, Sturzbecher-Hoehne M, Rupert PB, Dahlia An D, Illy MC, Ralston CY, Brabec J, Jong WAD, Strong RK, Abergel RJ (2017) Chelation and stabilization of berkelium in oxidation state +IV. Nat Chem 9:843
Deblonde J-P, Lohrey TD, Dahlia An D, Abergel RJ (2018) Toxic heavy metal—Pb, Cd, Sn—complexation by the octadentate hydroypyridinonate. New J Chem 42:7649–7658
Ansari SA, Pathak P, Mohapatra PK, Manchanda VK (2012) Chemistry of diglycolamides: promising extractants for actinide partitioning. Chem Rev 112:1751
Horwitz EP, McAlister DR, Bond AH, Barrans JRE (2005) Novel Extraction of chromatographic resins based on tetraalkyldiglycolamides: characterization and potential applications. Solvent Extr Ion Exch 23:319
Sasaki Y, Sugo Y, Suzuki S, Tachimori S (2001) The Novel extractants, Diglycolamides, for the extraction of lanthanides and actinides in HNO3-n-dodecane system. Solvent Extr Ion Exch 19(1):91–103
Lundberg D, Persson I (2016) The size of actinoid(III) ions – structural analysis vs. common misinterpretations. Coord Chem Rev 318:131–134
Radchenko V, Mastren T, Meyer CAL, Ivanov AS, Bryantsev VS, Copping R, Denton D, Engle JW, Griswold JR, Murphy K, Wilson JJ, Owens A, Wyant L, Birnbaum ER, Fitzsimmons J, Medvedev D, Cutler CS, Mausner LF, Nortier MF, John KD, Mirzadeh S, Fassbender ME (2017) Radiometric evaluation of Diglycolamide resins for the chromatographic separation of actinium from fission product lanthanides. Talanta 175:318–324
Radchenko V, Engle JW, Wilson JJ, Maassen JR, Nortier FM, Taylor WA, Birnbaum ER, Hudston LA, John KD, Fassbender ME (2015) Application of ion exchange and extraction chromatography to the separation of actinium from Proton-Irradiated thorium metal for analytical purposes. J Chromatogr A 1380:55–63
Sturzbecher-Hoehne M, Deblonde GJ-P, Abergel RJ (2013) Solution thermodynamic evaluation of hydroxypyridinonate chelators 3,4,3-LI(1,2-HOPO) and 5-LIO(Me-3,2-HOPO) for UO2(VI) and Th(IV) decorporation. Radiochim Acta 101:359–366
Sturzbecher-Hoehne M, Choi TA, Abergel RJ (2015) Hydroxypyridinonate complex stability of group (IV) metals and tetravalent f-Block elements: the key to the next generation of chelating agents for radiopharmaceuticals. Inorg Chem 54:3462–3468
Suliman G, Pommé S, Marouli M, Van Ammel R, Stroh H, Jobbágy V, Paepen J, Dirican A, Bruchertseifer F, Apostolidis C, Morgenstern A (2013) Half lives of 221Fr, 217At, 213Bi, 213Po and 209Pb from the 225Ac decay series. Appl Radiat Isot 77:32–37
Bertelsen ER, Jessica JA, Jenifer SC (2020) A Survey of extraction chromatographic f-element separations developed by E. P. Horwitz. Solvent Extr Ion Exch 38:251
Kelley PM, Deblonde J-PG, Su J, Booth HC, Abergel RJ, Batista RE, Yang P (2018) Bond covalency and oxidation state of actinide ions complexed with therapeutic chelating agent 3,4,3-LI(1,2-HOPO). Inorg Chem 57:5352–5536
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malmbeck, R., Banik, N.l. & Nicholl, A. DGA resin capacity factors for Ac, Am and Th under tetravalent actinide selective complexation. J Radioanal Nucl Chem 329, 1387–1392 (2021). https://doi.org/10.1007/s10967-021-07774-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07774-0