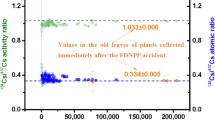
Abstract
The influence of extraction process on Cs isotope ratios was investigated by thermal ionization mass spectrometry (TIMS) for nitric-acid (HNO3) treatments of Fukushima Daiichi nuclear power plant accident-contaminated soil samples. In contrast to the consistent TIMS results for conc. HNO3 treatments at 90–200 °C, dilute HNO3 treatment provided a statistically significant 3.0‰-higher 135Cs/137Cs average isotope ratio than the conc. HNO3 treatment conducted at the same temperature. The higher isotope ratio is caused by a specific extraction process making detectable the deviated distribution of Cs isotopes in clay minerals or leading to matrix effects responsible for instrumental mass bias.



Similar content being viewed by others
References
Saito K, Tanihata I, Fujiwara M, Saito T, Shimoura S, Otsuka T, Onda Y, Hoshi M, Ikeuchi Y, Takahashi F, Kinouchi N, Saegusa J, Seki A, Takemiya H, Shibata T (2015) Detailed deposition density maps constructed by large-scale soil sampling for gamma-ray emitting radioactive nuclides from the Fukushima Dai-ichi nuclear power plant accident. J Environ Radioact 139:308–319
Kusakabe M, Oikawa S, Takata H, Misonoo J (2013) Spatiotemporal distributions of Fukushima-derived radionuclides in nearby marine surface sediments. Biogeosciences 10:5019–5030
Külahcı F, Bilici A (2019) Advances on identification and animated simulations of radioactivity risk levels after Fukushima nuclear power plant accident (with a data bank): a critical review. J Radioanal Nucl Chem 321:1–30
Unterweger MP, Hoppes DD, Schima FJ, Coursey JS (2009) Radionuclide half-life measurements data. Updated November 15, 2019. https://www.nist.gov/pml/radionuclide-half-life-measurements/radionuclide-half-life-measurements-data. Accessed 8 Feb 2021
Chaisan K, Smith JT, Bossew P, Kirchner G, Laptev GV (2013) Worldwide isotope ratios of the Fukushima release and early-phase external dose reconstruction. Sci Rep 3:2520
Xu S, Cook GT, Cresswell AJ, Dunbar E, Freeman SPHT, Hou X, Kinch H, Naysmith P, Sanderson DWC, Zhang L (2016) Carbon, cesium and iodine isotopes in Japanese cedar leaves from Iwaki, Fukushima. J Radioanal Nucl Chem 310:927–934
Ishida M, Yamazaki H (2017) Radioactive contamination in the Tokyo metropolitan area in the early stage of the Fukushima Daiichi nuclear power plant (FDNPP) accident and its fluctuation over five years. PLoS ONE 12:e0187687
Nishizawa Y, Yoshida M, Sanada Y, Torii T (2016) Distribution of the 134Cs/137Cs ratio around the Fukushima Daiichi nuclear power plant using an unmanned helicopter radiation monitoring system. J Nucl Sci Technol 53:468–474
Firestone RB, Baglin CM (1999) In: Baglin CM (ed), Chu SYF (ed, electronic content), Table of Isotopes, 8th edn, Wiley-VCH
Snyder DC, Delmore JE, Tranter T, Mann NR, Abbott ML, Olson JE (2012) Radioactive cesium isotope ratios as a tool for determining dispersal and re-dispersal mechanisms downwind from the Nevada nuclear security site. J Environ Radioact 110:46–52
Snow MS, Snyder DC (2016) 135Cs/137Cs isotopic composition of environmental samples across Europe: environmental transport and source term emission applications. J Environ Radioact 151:258–263
Snow MS, Snyder DC, Clark SB, Kelley M, Delmore JE (2015) 137Cs activities and 135Cs/137Cs isotopic ratios from soils at Idaho national laboratory: a case study for contaminant source attribution in the vicinity of nuclear facilities. Environ Sci Technol 49:2741–2748
Zheng J, Tagami K, Bu W, Uchida S, Watanabe Y, Kubota Y, Fuma S, Ihara S (2014) 135Cs/137Cs isotopic ratio as a new tracer of radiocesium released from the Fukushima nuclear accident. Environ Sci Technol 48:5433–5438
Shibahara Y, Kubota T, Fujii T, Fukutani S, Takamiya K, Konno M, Mizuno S, Yamana H (2017) Analysis of cesium isotope compositions in environmental samples by thermal ionization mass spectrometry–3. J Nucl Sci Technol 54:158–166
Shibahara Y, Kubota T, Fujii T, Fukutani S, Ohta T, Takamiya K, Okumura R, Mizuno S, Yamana H (2014) Analysis of cesium isotope compositions in environmental samples by thermal ionization mass spectrometry–1. A preliminary study for source analysis of radioactive contamination in Fukushima prefecture. J Nucl Sci Technol 51:575–579
Snow MS, Snyder DC, Delmore JE (2016) Fukushima Daiichi reactor source term attribution using cesium isotope ratios from contaminated environmental samples. Rapid Commun Mass Spectrom 30:523–532
Dunne JA, Richards DA, Chen HW (2017) Procedures for precise measurements of 135Cs/137Cs atom ratios in environmental samples at extreme dynamic ranges and ultra-trace levels by thermal ionization mass spectrometry. Talanta 174:347–356
Yang G, Tazoe H, Yamada M (2016) Rapid determination of 135Cs and precise 135Cs/137Cs atomic ratio in environmental samples by single-column chromatography coupled to triple-quadrupole inductively coupled plasma-mass spectrometry. Anal Chim Acta 908:177–184
Ohno T, Muramatsu Y (2014) Determination of radioactive cesium isotope ratios by triple quadrupole ICP-MS and its application to rainwater following the Fukushima Daiichi nuclear power plant accident. J Anal At Spectrom 29:347–351
Cao L, Zheng J, Tsukada H, Pan S, Wang Z, Tagami K, Uchida S (2016) Simultaneous determination of radiocesium (135Cs, 137Cs) and plutonium (239Pu, 240Pu) isotopes in river suspended particles by ICP-MS/MS and SF-ICP-MS. Talanta 159:55–63
Zheng J, Bu W, Tagami K, Shikamori Y, Nakano K, Uchida S, Ishii N (2014) Determination of 135Cs and 135Cs/137Cs atomic ratio in environmental samples by combining ammonium molybdophosphate (AMP)-selective Cs adsorption and ion-exchange chromatographic separation to triple-quadrupole inductively coupled plasma–mass spectrometry. Anal Chem 86:7103–7110
Zheng J, Cao L, Tagami K, Uchida S (2016) Triple-quadrupole inductively coupled plasma-mass spectrometry with a high-efficiency sample introduction system for ultratrace determination of 135Cs and 137Cs in environmental samples at femtogram levels. Anal Chem 88:8772–8779
Nishihara K, Iwamoto H, Suyama K (2012) Estimation of fuel compositions in Fukushima-Daiichi nuclear power plant. JAEA-Data/Code, No. 2012-018 (Japanese)
Bu W, Tang L, Liu X, Wang Z, Fukuda M, Zheng J, Aono T, Hu S, Wang X (2019) Ultra-trace determination of the 135Cs/137Cs isotopic ratio by thermal ionization mass spectrometry with application to Fukushima marine sediment samples. J Anal At Spectrom 34:301–309
Zhu L, Hou X, Qiao J (2020) Determination of ultralow level 135Cs and 135Cs/137Cs ratio in environmental samples by chemical separation and triple quadrupole ICP-MS. Anal Chem 92:7884–7892
Zhu L, Xu C, Hou X, Qiao J, Zhao Y, Liu G (2020) Determination of ultratrace level 135Cs and 135Cs/137Cs ratio in small volume seawater by chemical separation and thermal ionization mass spectrometry. Anal Chem 92:6709–6718
Parajuli D, Takahashi A, Tanaka H, Sato M, Fukuda S, Kamimura R, Kawamoto T (2015) Variation in available cesium concentration with parameters during temperature induced extraction of cesium from soil. J Environ Radioact 140:78–83
Snow MS, Snyder DC, Mann NR, White BM (2015) Method for ultra-trace cesium isotope ratio measurements from environmental samples using thermal ionization mass spectrometry. Int J Mass Spectrom 381–382:17–24
Higatsberger MJ, Demorest HL, Nier AO (1954) Secondary emission from Nichrome V, CuBe, and AgMg alloy targets due to positive ion bombardment. J Appl Phys 25:883–886
Shikamori Y, Nakano K (2015) Feasibility study on the analysis of radioisotopes: Sr-90 and Cs-137. In Agilent 8800 ICP-QQQ Application Handbook, 2nd edn, pp77–78
Sawhney BL (1972) Selective sorption and fixation of cations by clay minerals: a review. Clays Clay Miner 20:93–100
Fuller AJ, Shaw S, Ward MB, Haigh SJ, Mosselmans JFW, Peacock CL, Stackhouse S, Dent AJ, Trivedi D, Burke IT (2015) Caesium incorporation and retention in illite interlayers. Appl Clay Sci 108:128–134
Yamasaki S, Takeda A, Nanzyo M, Taniyama I, Nakai M (2001) Background levels of trace and ultra-trace elements in soils of Japan. Soil Sci Plant Nutr 47:755–765
Wilding MW (1961) Cesium removal from acidic radioactive waste solutions. U.S. Atomic Energy Commission (AEC) Research and Development Report, IDO-14544, Idaho Falls, Idaho
Mähler J, Persson I (2012) A study of the hydration of the alkali metal ions in aqueous solution. Inorg Chem 51:425–438
Smit JVR (1958) Ammonium salts of the heteropolyacids as cation exchangers. Nature 181:1530–1531
Amrhein V, Greenland S, McShane B (2019) Retire statistical significance. Nature 567:305–307
Amrhein V, Trafimow D, Greenland S (2019) Inferential statistics as descriptive statistics: there is no replication crisis if we don’t expect replication. Am Stat 73(S1):262–270
National Institute for Agro-Environmental Sciences. Research report 13th collection (1996) [Typology and regional features of clay mineral composition in the lowland soils of Japan] Wagakuni no teichidojyou ni okeru nendokoubutsu sosei no ruikeika to chiikiteki tokuchou (Japanese). http://www.naro.affrc.go.jp/archive/niaes/sinfo/result/result13/result13_02.html
Tanaka K, Watanabe N, Yamasaki S, Sakaguchi A, Fan Q, Takahashi Y (2018) Mineralogical control of the size distribution of stable Cs and radiocesium in riverbed sediments. Geochem J 52:173–185
Brouwer E, Baeyens B, Maes A, Cremers A (1983) Cesium and rubidium ion equilibria in illite clay. J Phys Chem 87:1213–1219
Bradbury MH, Baeyens B (2000) A generalised sorption model for the concentration dependent uptake of caesium by argillaceous rocks. J Contam Hydrol 42:141–163
Wick S, Baeyens B, Fernandes MM, Voegelin A (2018) Thallium adsorption onto illite. Environ Sci Technol 52:571–580
Mukai H, Hatta T, Kitazawa H, Yamada H, Yaita T, Kogure T (2014) Speciation of radioactive soil particles in the Fukushima contaminated area by IP autoradiography and microanalyses. Environ Sci Technol 48:13053–13059
Taylor VF, Evans RD, Cornett RJ (2008) Preliminary evaluation of 135Cs/137Cs as a forensic tool for identifying source of radioactive contamination. J Environ Radioact 99:109–118
Lee T, Ku TL, Lu HL, Chen JC (1993) First detection of fallout Cs-135 and potential applications of 137Cs/135Cs ratios. Geochim Cosmochim Acta 57:3493–3497
Krupskaya VV, Zakusin SV, Tyupina EA, Dorzhieva OV, Zhukhlistov AP, Belousov PE, Timofeeva MN (2017) Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions. Minerals 7:49
Turpault MP, Trotignon L (1994) The dissolution of biotite single crystals in dilute HNO3 at 24°C: evidence of an anisotropic corrosion process of micas in acidic solutions. Geochim Cosmochim Acta 58:2761–2775
Trubača-Boginska A, Ādiņa R, Vaivars G, Švirksts J (2018) A Study on acidification and intercalation of illite clay minerals and their potential use as a filler in SPEEK composite membranes. Key Eng Mater 762:186–191
Steudel A, Batenburg LF, Fischer HR, Weidler PG, Emmerich K (2009) Alteration of non-swelling clay minerals and magadiite by acid activation. Appl Clay Sci 44:95–104
Albarède F, Beard B (2004) Analytical methods for non-traditional isotopes. Rev Mineral Geochem 55:113–152
van Zuilen K, Müller T, Nägler TF, Dietzel M, Küsters T (2016) Experimental determination of barium isotope fractionation during diffusion and adsorption processes at low temperatures. Geochim Cosmochim Acta 186:226–241
White WM (2020) In: Geochemistry, 2nd edn, Chapter 9, Stable isotope geochemistry. John Wiley & Sons, pp 432–511, ISBN: 9781119438052
Sharp Z (2006) In: Principles of stable isotope geochemistry, Chapter 3, Equilibrium isotopic fractionation, Prentice Hall, pp 40–63, ISBN: 0130091391
Acknowledgements
The authors are grateful to Prof. Takeshi Ohno from Gakushuin University for his advice on ICP-MS/MS measurement conditions. We would also like to take this opportunity to thank Mr. Taichi Yamashita and Drs. Yasuhiko Fujii and Zenko Yoshida for great support and helpful advice in carrying out this research. This research is partially supported by "Support for Tokyo Tech Advanced Researchers (STAR) from Tokyo Institute of Technology".
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takahashi, H., Park, K.C., Nomura, M. et al. Influence of extraction process on Cs isotope ratios for Fukushima Daiichi nuclear power plant accident-contaminated soil. J Radioanal Nucl Chem 329, 327–336 (2021). https://doi.org/10.1007/s10967-021-07760-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07760-6