Abstract
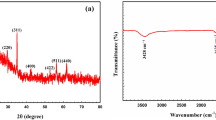
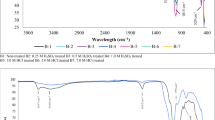
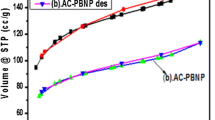
Since the Fukushima plant accident took place in 2011, a large amount of radioactive cesium ions have been released and their separation from wastewater has been a major concern. Herein, we have synthesized a Cs-137 adsorbent: calcium-doped Prussian Blue (Ca-PB), using a simple solution co-precipitation technique. The adsorbent was characterized by FTIR and XRD, and systematically evaluated the kinetics and adsorption equilibrium for Cs+ adsorbed to Ca-PB. The experimental data were followed by a pseudo-second order kinetic model and well adapted to Langmuir’s isothermal model. The research showed that Ca-PB can be used to treat water contaminated by Cs-137 in extremely acidic conditions.







Similar content being viewed by others
References
Hirose K (2012) 2011 Fukushima Dai-ichi nuclear power plant accident: summary of regional radioactive deposition monitoring result. J Environ Radioact 111:13–17
Steinhauser G, Brandl A, Johnson TE (2014) Comparison of the Chernobyl and Fukushima nuclear accidents: a review of the environmental impacts. Sci Total Environ 470–471:800–817
Buesseler K, Dai M, Aoyama M, Benitez-Nelson C, Charmasson S, Higley K, Maderich V, Masque P, Oughton D, Smith JN (2017) Fukushima Daiichi-derived radionuclides in the ocean: transport fate, and impacts. Ann Rev Mar Sci 9:173–203
Thammawong C, Opaprakasit P, Tangboriboonrat P, Sreearunothai P (2013) Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J Nanoparticle Res 15(6):1689. https://doi.org/10.1007/s11051-013-1689-z
Narayanam PK, Jishnu A, Sankaran K (2018) Graphene oxide supported filtration of cesium from aqueous systems. Coll Surf A Physicochem Eng Asp 539:416–243
Nayl AA, Ahmed IM, Abd-Elhamid AI, Aly HF, Attallah MF (2020) Selective sorption of 134Cs and 60Co radioisotopes using synthetic nanocopper ferrocyanide-SiO2 materials. Sep Purif Technol 234:116060. https://doi.org/10.1016/j.seppur.2019.116060
Olatunji MA, Khandaker MU, Mahmud HNME, Amin YM (2015) Influence of adsorption parameters on cesium uptake from aqueous solutions—a brief review. RSC Adv 5:71658–71683
Li WJ, Han C, Cheng G, Chou SL, Liu HK, Dou SX (2019) chemical properties, structural properties, and energy storage applications of Prussian blue analogues. Small 15(32):1900470. https://doi.org/10.1002/smll.201900470
Han F, Zhang GH, Gu P (2013) Adsorption kinetics and equilibrium modeling of cesium on copper ferrocyanide. J Radioanal Nucl Chem 295:369–377
Faustino PJ, Yang Y, Progar JJ, Brownell CR, Sadrieh N, May JC, Leutzinger E, Place DA, Duffy EP, Houn F, Loewke SA, Mecozzi VJ, Ellison CD, Khan MA, Hussain AS, Lyon RC (2008) Quantitative determination of cesium binding to ferric hexacyanoferrate: Prussian blue. J Pharm Biomed Anal 47(1):114–125
Fujita H, Sasano H, Miyajima R (2014) Adsorption equilibrium and kinetics of cesium onto insoluble Prussian blue synthesized by an immediate precipitation reaction between Fe3+ and [Fe(CN)6]4-. Adsorption 20:905–915
Torad NL, Hu M, Imura M, Naito M, Yamauchi Y (2012) Large Cs adsorption capability of nanostructured Prussian blue particles with high accessible surface areas. J Mater Chem 22:18261–18267
Ishizaki M, Akiba S, Ohtani A, Hoshi Y, Ono K, Matsuba M, Togashi T, Kananizuka K, Sakamoto M, Takahashi A, Kawamoto T, Tanaka H, Watanabe M, Arisaka M, Nankawad T, Kurihara M (2013) Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of Prussian blue filled with coordination and crystallization water molecules. Dalton Trans 42:16049–16055
Qin YH, Kang B, Dai YD, Li J, Shen WT (2014) Adsorption mechanism analysis of cesium ions nickel hexacyanoferrate. Atomic Energy Sci Technol 48:1751–1756
Ojwang DO, Grins J, Wardecki D, Valvo M, Renman V, Haggstrom L, Ericsson T, Gustafsson T, Mahmoud A, Hermann RP, Svensson G (2016) Structure characterization and properties of K-containing copper hexacyanoferrate. Inorg Chem 55:5924–5934
Buser HJ, Schwarzenbach D, Petter W, Ludi A (1977) The crystal structure of Prussian blue: Fe4[Fe(CN)6]3xH2O. Inorg Chem 16(11):2704–2710
Loos-Neskovic C, Ayrault S, Badillo V, Jimenez B, Garnier E, Fedoroff M, Jones DJ, Merinov B (2004) Structure of copper-potassium hexacyanoferrate (II) and sorption mechanisms of cesium. J Solid State Chem 177:1817–1828
Smiciklas I, Dimovic S, Plecas I (2007) Removal of Cs1+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite. Appl Clay Sci 35:139–144
Khandaker S, Toyohara Y, Saha GC, Awual MR, Kuba T (2020) Development of synthetic zeolites from bio-slag for cesium adsorption:Kinetic, isotherm and thermodynamic studies. J Water Process Eng 33:101055. https://doi.org/10.1016/j.jwpe.2019.101055
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Crittenden JC, Sanongraj S, Bulloch JL, Hand DW, Rogers TN, Speth TF, Ulmer M (1999) Correlation of aqueous-phase adsorption isotherms. Environ Sci Technol 33:2926–2933
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1362–1403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Kasap S, Tel H, Piskin S (2011) Preparation of TiO2 nanoparticles by sonochemical method, isotherm, thermodynamic and kinetic studies on the sorption of strontium. J Radioanal Nucl Chem 289:489–495
El-Naggar IM, Zakaria ES, Ali IM, Khalil M, El-Shahat MF (2012) Kinetic modeling analysis for the removal of cesium ions from aqueous solutions using polyaniline titanotungstate. Arab J Chem 5:109–119
Acknowledgements
The authors would like to thank anonymous reviewers for their valuable comments. The work is supported in part by the General Project of Scientific Research Fund of Hunan Provincial Education Department (grant number 18C0461).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, T., Xia, L. Kinetic and equilibrium studies of Cs-137 sorption on calcium-doped Prussian blue. J Radioanal Nucl Chem 328, 1011–1018 (2021). https://doi.org/10.1007/s10967-021-07742-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07742-8