Abstract
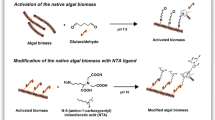
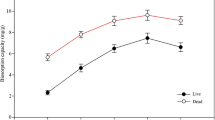
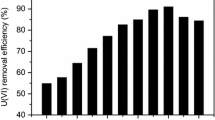
In this study, the native and 2,5-diaminobenzene sulfonic acid (DABSA) attached Lentinus concinnus biomasses were utilized for removal of U(VI) ions from solutions. At 25 °C, the maximum uranium ion adsorption capacity of the native and DABSA attached fungal biomasses were found to be 118.6 and 539.2 mg/g, respectively, at pH 6.0. The negative ΔG° values of U(VI) removal showed that the adsorption procedure were spontaneous. ATR-FTIR data showed that U(VI) ions adsorption the adsorbents was mainly attributed by amine, carboxyl and sulfate groups.











Similar content being viewed by others
References
Zhu W, Liu Z, Chen L, Dong Y (2011) Sorption of uranium(VI) on Na-attapulgite as a function of contact time, solid content, pH, ionic strength, temperature and humic acid. J Radioanal Nucl Chem 289(3):781–788
Liang L, Lin X, Liu Y, Sun S, Chu H, Chen Y, Liu D, Luo X, Zhang J, Shang R (2020) Carboxymethyl konjac glucomannan mechanically reinforcing gellan gum microspheres for uranium removal. Inter J Biol Macromol 145:535–546
Ozturk M, Zorer OS, Gulcan M (2021) Synthesis and characterization of UTSA-76 metal organic framework containing Lewis basic sites for the liquid-phase adsorption of U(VI). Colloid Surf A 609:125663
Kolhe N, Zinjarde S, Acharya C (2020) Removal of uranium by immobilized biomass of a tropical marine yeast Yarrowia lipolytica. J Environ Radioact 223–224:106419
Zhou Y, Li Y, Liu D, Wang X, Liu D, Xu L (2020) Synthesis of the inorganic-organic hybrid of two-dimensional polydopamine-functionalized titanate nano-sheets and its efficient extraction of U(VI) from aqueous solution. Colloid Surf A 607:125422
Sun Y, Kang Y, Zhong W, Liu Y, Dai Y (2020) A simple phosphorylation modification of hydrothermally cross-linked chitosan for selective and efficient removal of U(VI). J Solid State Chem 292:121731
Tuzen M, Saleh TA, Sarı A, Naeemullah (2020) Interfacial polymerization of trimesoyl chloridewith melamine and palygorskite for efficient uranium ions ultra-removal. Chem Eng Res Des 159:353–361
Bai Z, Liu Q, Song D, Zhang H, Liu J, Chen R, Yu J, Li R, Wang J (2020) Preparation of a 3D multi-branched chelate adsorbent for high selective adsorption of uranium(VI): Acrylic and diaminomaleonitrile functionalized waste hemp fiber. React Funct Polym 149:104512
Tang N, Liang J, Niu C, Wang H, Luo Y, Xing W, Ye S, Liang C, Guo H, Guo J, Zhang Y, Zeng G (2020) Amidoxime-based materials for uranium recovery and removal. J Mater Chem A 8:7588
Mohammed AAR (2020) Potentiality of quercetin-sodium hydroxide modified SpirulinaPlatensis in uranium biosorption from waste effluent. Int J Environ Stud 77:48–60
Bayramoglu G, Arica MY (2019) Star type polymer grafted and polyamidoxime modified silica coated-magnetic particles for adsorption of U(VI) ions from solution. Chem Eng Res Des 147:146–159
Singhal P, Vats BG, Pulhani V (2020) Magnetic nanoparticles for the recovery of uranium from sea water: Challenges involved from research to development. J Ind Eng Chem 90:17–35
Bai J, Ma X, Yan H, Zhu J, Wang K, Wang J (2020) A novel functional porous organic polymer for removal of uranium from wastewater. Micropor Mesopor Mater 306:110441
Huang Y, Zheng H, Li H, Zhao C, Zhao R, Li S (2020) Highly selective uranium adsorption on 2-phosphonobutane-1,2,4-tricarboxylic acid-decorated chitosan-coated magnetic silica nanoparticles. Chem Eng J 388:124349
Puspitasari T, Darwis D, Pangerteni DS, Seftiani S, Nurhasni N (2020) Synthesis and characterization of zeolite–amo hybrid composite adsorbent by using simultaneous gamma irradiation for uranium (VI) removal from aqueous solutions. Macromol Symp 391:1900156
Chen L, Ning S, Huang Y, Chen Y, Ju Z, He X, Lu L, Zhou H, Wang X, Wu Y, Wei Y (2020) Effects of speciation on uranium removal efficiencies with polyamine functionalized silica composite adsorbent in groundwater. J Clean Produc 256:120379
Hamza MF, Mubark AE, Wei Y, Vincent T, Guibal E (2020) Quaternization of composite algal/PEI beads for enhanced uranium sorption—application to ore acidic leachate. Gels 6:12
Bayramoglu G, Akbulut A, Acıkgoz-Erkaya I, Arica MY (2018) Uranium sorption by native and nitrilotriacetate-modified Bangia atropurpurea biomass: kinetics and thermodynamics J Appl Phycol 30:649–661
Bayramoglu G, Arica MY (2017) Polyethylenimine and tris(2-aminoethyl)amine modified p(GMA-co-EGDMA) microbeads for adsorption of U(VI) ions: Equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 312:293–303
Ahmad M, Wang J, Yang Z, Zhang Q, Zhang B (2020) Ultrasonic-assisted preparation of amidoxime functionalized silica framework via oil-water emulsion method for selective uranium adsorption. Chem Eng J 389:124441
Janu VC, Meena RK, Kumar N, Sharma RK (2020) Surface fluorinated hematite for uranium removal from radioactive effluent. J Environ Chem Eng 8:104218
Amesh P, Suneesh AS, Venkatesan KA, Chandra M, Ravindranath NA (2020) High capacity amidic succinic acid functionalized mesoporous silica for the adsorption of uranium. Colloid Surf A 602:125053
Boulanger N, Kuzenkova AS, Iakunkov A, Romanchuk AY, Trigub AL, Egorov AV, Bauters S, Amidani L, Retegan M, Kvashnina KO, Kalmykov SN, Talyzin AV (2020) Enhanced sorption of radionuclides by defect-rich graphene oxide. ACS Appl Mater Interfaces 12:45122–45135
Yousef LA, Bakry AR, Abd El-Magied MO (2020) Uranium (VI) recovery from its leach liquor using zirconium molybdophosphate composite: kinetic, equilibrium and thermodynamic studies. J Radioanal Nucl Chem 323:549–556
Khan A, Wei D, Khuda F, Ma R, Ismail M, Ai Y (2020) Comparative adsorption capabilities of rubbish tissue paper–derived carbon-doped MgO and CaCO3 for EBT and U(VI), studied by batch, spectroscopy and DFT calculations. Environ Sci Pollut Res 27:13114–13130
Huo Z, Zhao S, Yi J, Zhang H, Li J (2020) Biomass-based cellulose functionalized by phosphonic acid with high selectivity and capacity for capturing U(VI) in aqueous aolution. Appl Sci 10:5455
Arica MY, Bayramoglu G (2016) Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. J Radioanal Nucl Chemy 310:711–724
Bayramoglu G, Arica MY (2016) Amidoxime functionalized Trametes trogii pellets for removal of uranium (VI) from aqueous medium. J Radioanal Nucl Chem 307:373–384
Hamza MF, Gamal A, Hussein G, Ngar MS, Abdel-Rahman AA-H, Wei Y, Guibal E (2019) Uranium(VI) and zirconium(IV) sorption on magnetic chitosan derivatives – effect of different functional groups on separation properties. J Chem Technol Biotechnol 94:3866–3882
Zhang W, Dong F, Liu M, Song H, Nie X, Huo T, Zhao Y, Wang P, Qin Y, Zhou L (2020) Reduction and enrichment of uranium after biosorption on inactivated Saccharomyces cerevisiae. Pol J Environ Stud 29:1461–1472
Bayramoglu G, Arica MY (2016) MCM-41 silica particles grafted with polyacrylonitrile: Modification in to amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Micropor Mesopor Mater 226:117–124
Bayramoglu G, Akbulut A, Arica MY (2015) Study of polyethyleneimine and amidoxime functionalized hybrid biomass of Spirulina (Arthrospira) platensis for adsorption of uranium (VI) ion. Environ Sci Pollut Res 22:17998–18010
Yousef LA, Bakry AR, Ahmad AA (2020) Uranium(VI) recovery from acidic leach liquor using manganese oxidecoated zeolite (MOCZ) modified with amine. J Radioanal Nucl Chem 324:409–421
Chaudharya M, Singhb L, Rekhac P, Srivastavab VC, Mohanty P (2019) Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine. Chem Eng J 378:122236
Yousef LA, Bakry AR, Ahmad AA (2020) Uranium(VI) adsorption using a mixture of 1-amino-2-naphthol-4-sulfonic acid and bentonite: kinetic and equilibrium studies. Radiochemistry 62:511–523
Bayramoglu G, Yilmaz M (2018) Azo dye removal using free and immobilized fungal biomasses: isotherms, kinetics and thermodynamic studies. Fibers and Polymers 19:877–886
Perez-Bustamante JA, Delgado FP (1971) The extraction and spectrophotometric determination of sexavalent uranium with arsenazo11 in aqueous-organic media. Analyst 96:407422
Bayramoglu G, Arica MY (2021) Strong and weak cation-exchange groups generated cryogels films for adsorption and purification of lysozyme. Food Chem 342:128295
Fang C, Tao Q, Dai Y (2020) Amidoximated orange peel as a specific uranium scavenger. J Radioanal Nucl Chem 326:1831–1841
Al-Anber MA, Al-Momani IF, Zaitoun MA, Al-Qaisi W (2020) Inorganic silica gel functionalized tris(2-aminoethyl)amine moiety for capturing aqueous uranium (VI) ion. J Radioanal Nucl Chem 325:605–623
Nuhanovic M, Grebo M, Draganovic S, Memic M, Smjecanin N (2019) Uranium(VI) biosorption by sugar beet pulp: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 322:2065–2078
Sabanovic E, Muhic-Sarac T, Nuhanovic M, Memic M (2019) Biosorption of uranium (VI) from aqueous solution by Citrus limon peels: kinetics, equilibrium and batch studies. J Radioanal Nucl Chem 319:425–435
Hamza MF, Wei Y, Benettayeb A, Wang X, Guibal E (2020) efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micromagnetite chitosan derivative. J Mater Sci 55:4193–4212
Liu L, Lin X, Li M, Chu H, Wang H, Xie Y, Du Z, Liu M, Liang L, Gong H, Zhou J, Li Z, Luo X (2021) Microwave-assisted hydrothermal synthesis of carbon doped with phosphorus for uranium(VI) adsorption. J Radioanal Nucl Chem 327:73–89
Ang KL, Li D, Nikoloski AN (2017) The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 1. Anionic and cationic resins Hydrometallurgy 174:147–155
Wang J, Yang S, Cheng G, Gu P (2020) The adsorption of europium and uranium on the sodium dodecyl sulfate modified molybdenum disulfide composites. J Chem Eng Data 65:2178–2185
Arica TA, Ayas E, Arica MY (2017) Magnetic MCM-41 silica particles grafted with poly (glycidylmethacrylate) brush: Modification and application for removal of direct dyes. Micropor Mesopor Mater 243:64–175
Dolatyari L, Shateri M, Yaftian MR, Rostamnia S (2019) Unmodified SBA-15 adsorbents for the removal and separation of Th(IV) and U(VI) ions: the role of pore channels and surface-active sites. Sep Sci Technol 54:2863–2878
Gado M, Atia B, Morcy A (2019) The role of graphene oxide anchored 1-amino-2-naphthol-4-sulphonic acid on the adsorption of uranyl ions from aqueous solution: kinetic and thermodynamic features. Int J EnvironAnal Chem 99:996–1015
Gao Z, Bandosz TJ, Zhao Z, Han M, Qiu J (2009) Investigation of factors affecting adsorption of transition metals on oxidized carbon nanotubes. J Hazard Mater 167:357–365
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos IA (2019) Critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434
Escudero C, Poch J, Villaescusa I (2013) Modelling of breakthrough curves of single and binary mixtures of Cu(II), Cd(II), Ni(II) and Pb(II) onto grape stalks waste. Chem Eng J 1217:129–138
Bayramoglu G, Arica MY (2016) Amidoxime functionalized Trametes trogii pellets for removal of uranium(VI) from aqueous medium. J Radioanal Nucl Chem 307:373–384
Fang C, Tao Q, Dai Y (2020) Amidoximated orange peel as a specifc uranium scavenger. J Radioanal Nucl Chem 326:1831–1841
Han J, Hu L, He L, Ji K, Liu Y, Chen C, Luo X, Tan N (2020) Preparation and uranium (VI) biosorption for tri-amidoxime modified marine fungus material. Environ Sci Pollut Res 27:37313–37323
Hamza MF, Roux JC, Guibal E (2018) Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem Eng J 344:124–137
Liu S, Ouyang J, Luo J, Sun L, Huang G, Ma J (2018) Removal of uranium(VI) from aqueous solution using graphene oxide functionalized with diethylenetriamine pentaacetic phenylenediamine. J Nucl Sci Technol 55:781–791
Li L, Tang S, Cheng B, Liao Q, Lu W, Dai Z, Tan Y, Sun J (2018) Synthesis and adsorption characteristics of calix[6]arene derivative modified Aspergillus niger-Fe3O4 bio-nanocomposite for U(VI). J Radioanal Nucl Chem 316:331–339
Ai L, Luo X, Lin X, Zhang S (2013) Biosorption behaviors of uranium (VI) from aqueous solution by sunflower straw and insights of binding mechanism. J Radioanal Nucl Chem 298:1823–1834
Acknowledgements
The authors on behalf of National Institute of Agrobiological Sciences (NIAS) wish to offer many thanks for supplying the white rot fungus (Lentinus concinnus).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
CRediT authorship contribution statement
Omur Celikbical: Methodology, Investigation, Formal analysis. Gulay Bayramoglu: Conceptualization, Methodology, Investigation, Validation, Writing—original draft, Funding acquisition. Ilkay Acıkgoz-Erkaya: Methodology, Visualization, Investigation. Mehmet Yakup Arica: Project administration, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Competing of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Celikbıcak, O., Bayramoglu, G., Acıkgoz-Erkaya, I. et al. Aggrandizement of uranium (VI) removal performance of Lentinus concinnus biomass by attachment of 2,5-diaminobenzenesulfonic acid ligand. J Radioanal Nucl Chem 328, 1085–1098 (2021). https://doi.org/10.1007/s10967-021-07708-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07708-w