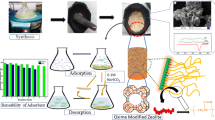
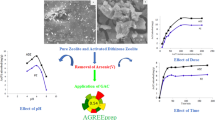
Abstract
Amidoxime (AO) based adsorbents have been proposed as low cost and efficient adsorbent for U(VI). Herein, we present a simple strategy for preparing AO modified artificial zeolite. The composition and morphology of the materials have been confirmed via XRD, FT-IR, TGA and SEM. The AO-artificial zeolite was synthesised as an efficient adsorbent for adsorbing U(VI). The critical factors affecting U(VI) adsorption from aqueous solution were exploited, such as pH, contact time, temperature and adsorbent dosage. This study reveals AO-artificial zeolite, along with a low-cost, environmentally friendly and facile synthesis, can be regarded as a promising material for uranium-containing wastewater treatment.









Similar content being viewed by others
References
Lv Z, Wang H, Chen C, Yang S, Chen L, Alsaedi A, Hayat T (2019) Enhanced removal of uranium(VI) from aqueous solution by a novel Mg–MOF-74-derived porous \({\text{MgO}}\)/carbon adsorbent. J Colloid Interface Sci 537:1–10
Su S, Liu Q, Liu J, Zhang H, Li R, Jing X, Wang J (2018) Polyethyleneimine-functionalized Luffa cylindrica for efficient uranium extraction. J Colloid Interface Sci 530:538–546
Duan S, Xu X, Liu X, Wang Y, Hayat T, Alsaedi A, Meng Y, Li J (2018) Highly enhanced adsorption performance of U(VI) by non-thermal plasma modified magnetic \({\text{ Fe}}_{3}{{\rm O}}_{{4}}\) nanoparticles. J Colloid Interface Sci 513:92–103
Duan S, Liu X, Wang Y, Shao D, Alharbi NS, Alsaedi A, Li J (2016) Highly efficient entrapment of U(VI) by using porous magnetic \({\text{Ni}}_{0.6}{\hbox{Fe}}_{2.4}{\rm{O}}_{4}\) micro-particles as the adsorbent. J Taiwan Inst Chem Eng 65:367–377
Lapka JL, Paulenova A, Alyapyshev MY, Babain VA, Herbst RS, Law JD (2009) Extraction of uranium(VI) with diamides of dipicolinic acid from nitric acid solutions. Radiochimica Acta 97(6):291–296
Tsaoulidis D, Ortega EG, Angeli P (2018) Intensified extraction of uranium(VI) in impinging-jets contactors. Chem Eng J 342:251–259
Pidchenko I, Kvashnina KO, Yokosawa T, Finck N, Bahl S, Schild D, Polly R, Bohnert E, Rossberg A, Gottlicher J et al (2017) Uranium redox transformations after U(VI) coprecipitation with magnetite nanoparticles. Environ Sci Technol 51(4):2217–2225
Rezaee M, Khalilian F (2015) Determination of uranium in water samples using homogeneous liquid–liquid microextraction via flotation assistance and inductively coupled plasma-optical emission spectrometry. J Radioanal Nuclear Chem 304(3):1193–1200
Gao MW, Zhu GR, Wang XH, Wang P, Gao CJ (2015) Preparation of short channels SBA-15-PVC membrane and its adsorption properties for removal of uranium(VI). J Radioanal Nuclear Chem 304(2):675–682
Li P, Wang J, Wang Y, Liang J, He B, Pan D, Fan Q, Wang X (2019) Photoconversion of U(VI) by \({\text{ TiO}}_2\): an efficient strategy for seawater uranium extraction. Chem Eng J 365:231–241
Wang X, Ding C, Xiangxue C, Wencai S, Yubing Z (2016) Adsorption of U(VI) on sericite in the presence of bacillus subtilis: a combined batch, EXAFS and modeling techniques. Geochimica et Cosmochimica Acta: J Geochem Soc Meteorit Soc 180:51–65
Hu T, Ding S, Deng H (2016) Application of three surface complexation models on U(VI) adsorption onto graphene oxide. Chem Eng J 289:270–276
Zhu K, Lu S, Gao Y, Zhang R, Tan X, Chen C (2017) Fabrication of hierarchical core–shell polydopamine\({\hbox{@}}\)\({\text{ MgAl }}-{\text{ LDH}}_s\) composites for the efficient enrichment of radionuclides. Appl Surf Sci 396:1726–1735
Yu X, Helvenston EM, Shuller-Nickles LC, Powell BA (2016) Surface complexation modeling of Eu(III) and U(VI) interactions with graphene oxide. Environ Sci Technol 50:1821–1827
Chen H, Chen Z, Zhao G, Zhang Z (2018) Enhanced adsorption of U(VI) and \(^{241}\) AM(III) from wastewater using \({\text{ Ca }}/{\text{ Al }}\) layered double hydroxide@carbon nanotube composites. J Hazard Mater 347:67–77
Yue Y, Mayes RT, Kim J, Fulvio PF, Sun XG, Tsouris C, Chen J, Brown S, Dai S (2013) Seawater uranium sorbents: preparation from a mesoporous copolymer initiator by atom-transfer radical polymerization. Angewandte Chemie Int Ed 52(50):13458–13462
Yuan D, Long C, Xin X, Yuan L, Liao S, Wang Y (2015) Removal of uranium(VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheres prepared by a controlled radical polymerization in the presence of DPE. Chem Eng J 285:358–367
Tan L, Liu Q, Jing X, Liu J, Song J, Hu S, Liu L, Wang J (2015) Removal of uranium(VI) ions from aqueous solution by magnetic cobalt ferrite/multiwalled carbon nanotubes composites. Chem Eng J 273:307–315
Yang W, Bai ZQ, Shi WQ, Yuan LY, Tian T (2013) MOF-76: from a luminescent probe to highly efficient U(VI) sorption material. Chem Commun 49:10415–10417
Zhao Y, Wang X, Li J, Wang X (2015) Amidoxime functionalization of mesoporous silica and its high removal of U(VI). Polym Chem 6:5376–5384
Xiao J, Jing Y, Yao Y, Wang X, Jia Y (2019) Synthesis of amidoxime-decorated 3D cubic mesoporous silica via self-assembly co-condensation as a superior uranium(VI) adsorbent. J Mol Liq 277:843–855
Ladshaw AP, Wiechert AI, Sadananda D, Sotira Y, Costas T (2017) Amidoxime polymers for uranium adsorption: influence of comonomers and temperature. Materials 10:1268
Wang Y, Wang Z, Ang R, Yang J, Liu N, Liao J, Yang Y, Tang J (2015) Synthesis of amidoximated graphene oxide nanoribbons from unzipping of multiwalled carbon nanotubes for selective separation of uranium(VI). RSC Adv 5:89309–89318
Zhang Z, Dong Z, Wang X, Ying D, Niu F, Cao X, Wang Y, Hua R, Liu Y, Wang X (2018) Ordered mesoporous polymer–carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chem Eng J 341:208–217
Xingjun W, Guojia J, Guiru Z, Chenghao S, Han Z, Congjie G (2019) Surface hydroxylation of SBA-15 via alkaline for efficient amidoxime-functionalization and enhanced uranium adsorption. Sep Purif Technol 209:623–635
Peng W, Huang G, Yang S, Guo C, Shi J (2019) Performance of biopolymer/graphene oxide gels for the effective adsorption of U(VI) from aqueous solution. J Radioanal Nuclear Chem 322(2):861–868
Wang Z, Zhao D, Wu C, Chen S, Wang Y, Chen C (2020) Magnetic metal organic frameworks/graphene oxide adsorbent for the removal of U(VI) from aqueous solution. Appl Radiat Isot 162:109160
Li W, Liu Q, Liu J, Zhang H, Li R, Li Z, Jing X, Wang J (2017) Removal U(VI) from artificial seawater using facilely and covalently grafted polyacrylonitrile fibers with lysine. Appl Surf Sci 403:378–388
Hai NT, You SJ, Hosseini-Bandegharaei A, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
We X, Liu Q, Zhang H, Liu J, Wang J (2017) Rapid and efficient uranium(VI) capture by phytic acid/polyaniline/ FeOOH composites. J Colloid Interface Sci 511:1–11
Guibal E, Milot C, Tobin JM (1998) Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Indus Eng Chem Res 37(4):1454–1463
Freundlich H (1907) Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie 57(1):385–470
Yang P, Liu Q, Liu J, Chen R, Li R, Bai X, Wang J (2018) Highly efficient immobilization of uranium(VI) from aqueous solution by phosphonate-functionalized dendritic fibrous nanosilica (DNFS). J Hazard Mater 363:248–257
Dabrowski A (2001) Adsorption-from theory to practice. Adv Colloid Interface Sci 93:135–224
Duan S, Xu X, Liu X, Wang Y, Li J (2017) Highly enhanced adsorption performance of U(VI) by non-thermal plasma modified magnetic \({\text{ Fe}}_{3}{{\rm O}}_{4}\) nanoparticles. J Colloid Interface Sci 513:92–103
Imam EA, Elsayed I, Mahfouz MG, Tolba AA, Akashi T, Galhoum AA, Guibal E (2018) Synthesis of \(\alpha\)-aminophosphonate functionalized chitosan sorbents: effect of methyl versus phenyl group on uranium sorption. Chem Eng J 352:1022–1034
Zhang Z, Duan S, Chen H, Fengsong Z, Hayat T, Ahmed A, Jiaxing L (2018) Synthesis of porous magnetic \({\text{ Ni}}_{0.6}{\hbox{Fe}}_{2.4}{\hbox{O}}_{4}\) nanorods for highly efficient adsorption of U(VI). J Chem Eng Data ACS J Data 63(5): 1810–1820
Wang XK, Chen CL, Zhou X, Tan XL, Hu WP (2005) Diffusion and sorption of U(VI) incompacted bentonite studied by a capillary method. Radiochim Acta 93:273–278
Lu Z, Yalou S, Lijuan S, Xiangchen S, Suwen C, Wangsuo W (2016) Dihydroxy bezladely derivatives functionalized mesoporous silica SBA-15 for the sorption of U(VI). J Radioanal Nuclear Chem 310:125–137
Padmavathy KS, Madhu G, Haseena PV (2016) A study on effects of pH, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr(VI)) from wastewater by magnetite nanoparticles. Proc Technol 24:585–594
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47(17):9904–9910
Nakkeeran E, Selvaraju N (2017) Biosorption of chromium(VI) in aqueous solutions by chemically modified strychnine tree fruit shell. Int J Phytoremediation 19(12):1065–1076
Saini AS, Melo JS (2013) Biosorption of uranium by melanin: kinetic, equilibrium and thermodynamic studies. Bioresour Technol 149:155–162
Cejka J (1999) Infrared spectroscopy and thermal analysis of the uranyl minerals. Rev Mineral Geochem 38:520–622
Tavengwa Nikita Tawanda, Cukrowska E, Chimuka L (2014) Preparation, characterization and application of \({\text{ NaHCO}}_3\) leached bulk U(VI) imprinted polymers endowed with \(\gamma\)-MPS coated magnetite in contaminated water. J Hazard Mater 267:221–228
Sadeghi S, Azhdari H, Arabi H, Moghaddam AZ (2012) Surface modified magnetic \({\text{ Fe}}_3{{\rm O}}_{4}\) nanoparticles as a selective sorbent for solid phase extraction of uranyl ions from water samples. J Hazard Mater 215–216:208–216
Mahramanlioglu M (2003) Adsorption of uranium on adsorbents produced from used tires. J Radioanal Nuclear Chem 256(1):99–105
Camacho LM, Parra RR, Deng S (2011) Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater 189(1–2):286–293
Abdi MR, Shakur HR, Saraee KRE, Sadeghi M (2014) Effective removal of uranium ions from drinking water using CuO/X zeolite based nanocomposites: effects of nano concentration and cation exchange. J Radioanal Nuclear Chem 300(3):1217–1225
Liu HJ, Jing PF, Liu XY, Du KJ, Sun YK (2016) Synthesis of \(\beta\)-cyclodextrin functionalized silica gel and its application for adsorption of uranium(VI). J Radioanal Nuclear Chem 310(1):1–8
Das D, Sureshkumar MK, Koley S, Mithal N, Pillai CGS (2010) Sorption of uranium on magnetite nanoparticles. J Radioanal Nuclear Chem 285:447–454
Yusan S, Aslani MAA, Turkozu DA, Aycan HA, Aytas S, Akyil S (2010) Adsorption and thermodynamic behaviour of U(VI) on the Tendurek volcanic tuff. J Radioanal Nuclear Chem 283:231–238
Zou W, Zhao L, Han R (2011) Adsorption characteristics of uranyl ions by manganese oxide coated sand in batch mode. J Radioanal Nuclear Chem 288:239–249
Caykara T, Alaslan SS, Inam R (2010) Competitive adsorption of uranyl ions in the presence of Pb(II) and Cd(II) ions by poly(glycidyl methacrylate) microbeads carrying amidoxime groups and polarographic determination. J Appl Polym Ence 104:4168–4172
Acknowledgements
The authors will thank Xu Zhang for the help in characterization analysis. The authors gratefully acknowledge support from the Foundation of Heilongjiang Postdoctoral Science Foundation (LBH-Z17050), the China Postdoctoral Science Foundation (2019M651257), the Fundamental Research Funds for the Central Universities (3072020CF1501), and the Decommissioning of Nuclear Facilities and Special Funds for Radioactive Waste Management ([2017]955), the Innovation Funds of the Innovation Center of Nuclear Materials for National Defense Industry (ICNM-2020-ZH-15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, P., Yu, Q., Zhang, X. et al. Removal of U(VI) from aqueous solution using AO-artificial zeolite. J Radioanal Nucl Chem 327, 39–47 (2021). https://doi.org/10.1007/s10967-020-07485-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07485-y