Abstract
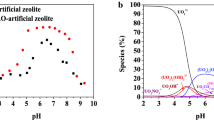
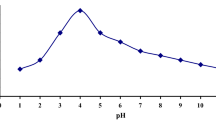
This paper reports synthesis of oxime modified zeolite-A as an efficient adsorbent for uranium (U(VI)). The as prepared adsorbent was thoroughly characterised and the effect of various experimental parameters were also studied. Maximum adsorption occurs in the pH range of 4–6 with adsorption efficiency of 98%. Adsorption experiment results showed that the equilibrium data fitted well to Langmuir model and follow pseudo second order kinetics. More importantly the spent adsorbent was successfully regenerated and multicycles of adsorption and desorption were showing good efficiency in each run. The material is stable and reusable up to 8 cycles with constant adsorption efficiency. The adsorption capacity of 4.92 mg/g is observed for U(VI) in presence of other competing metal ions which include Cr, Cd, Co, Pb and Mn. It can be inferred from the results that oxime modified zeolite-A is a promising adsorbent for recovery of U(VI) due its easy separation, high adsorption and excellent reusability.
Graphic abstract











Similar content being viewed by others
References
Brook BW, Alonso A, Meneley DA et al (2014) Why nuclear energy is sustainable and has to be part of the energy mix. Sustain Mater Technol 1:8–16. https://doi.org/10.1016/j.susmat.2014.11.001
Burns PC, Ewing RC, Navrotsky A (2012) Nuclear fuel in a reactor accident. Science 80(335):1184–1188. https://doi.org/10.1126/science.1211285
Waseem A, Ullah H, Rauf MK, Ahmad I (2015) Distribution of natural uranium in surface and groundwater resources: a review. Crit Rev Environ Sci Technol 45:2391–2423. https://doi.org/10.1080/10643389.2015.1025642
Bajwa BS, Kumar S, Singh S et al (2017) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10:13–19. https://doi.org/10.1016/j.jrras.2015.01.002
Quality D (2005) Uranium in drinking-water
Katsoyiannis IA, Zouboulis AI (2013) Removal of uranium from contaminated drinking water: a mini review of available treatment methods. Desalin Water Treat 51:2915–2925. https://doi.org/10.1080/19443994.2012.748300
Sun Y, Wu ZY, Wang X et al (2016) Macroscopic and microscopic investigation of U(VI) and Eu(III) adsorption on carbonaceous nanofibers. Environ Sci Technol 50:4459–4467. https://doi.org/10.1021/acs.est.6b00058
Abubakar M, Tamin MN, Saleh MA et al (2016) Preparation and characterization of a nigerian mesoporous clay-based membrane for uranium removal from underground water. Ceram Int 42:8212–8220. https://doi.org/10.1016/j.ceramint.2016.02.031
Ren B, Fan M, Tan L et al (2016) Electrospinning synthesis of porous Al2O3 nanofibers by pluronic P123 triblock copolymer surfactant and properties of uranium (VI)-sorption. Mater Chem Phys 177:190–197. https://doi.org/10.1016/j.matchemphys.2016.04.017
Oyola Y, Dai S (2016) High surface-area amidoxime-based polymer fibers co-grafted with various acid monomers yielding increased adsorption capacity for the extraction of uranium from seawater. Dalt Trans 45:8824–8834. https://doi.org/10.1039/c6dt01114d
Wang R, Ye J, Ning G et al (2016) Microwave-assisted rapid synthesis of cerium phosphates and their adsorption on uranium(VI). Res Chem Intermed 42:5013–5025. https://doi.org/10.1007/s11164-015-2342-5
Mustafa J, Kausar A, Bhatti HN, Ilyas S (2016) Sequestering of uranium(VI) onto eucalyptus bark: kinetic, equilibrium and thermodynamic studies. Desalin Water Treat 57:14578–14589. https://doi.org/10.1080/19443994.2015.1065443
Olmez Aytas S, Akyil S, Eral M (2004) Adsorption and thermodynamic behavior of uranium on natural zeolite. J Radioanal Nucl Chem 260:119–125. https://doi.org/10.1023/B:JRNC.0000027070.25215.92
Matijasevic S, Zildzovic S, Stojanovic J et al (2016) Removal of uranium(VI) from aqueous solution by acid modified zeolites. Zast Mater 57:551–558. https://doi.org/10.5937/zasmat1604551m
Nekhunguni PM, Tavengwa NT, Tutu H (2017) Sorption of uranium(VI) onto hydrous ferric oxide-modified zeolite: assessment of the effect of pH, contact time, temperature, selected cations and anions on sorbent interactions. J Environ Manage 204:571–582. https://doi.org/10.1016/j.jenvman.2017.09.034
Liu F, Xiong W, Liu J et al (2018) Novel amino-functionalized carbon material derived from metal organic framework: a characteristic adsorbent for U(VI) removal from aqueous environment. Colloids Surf A Physicochem Eng Asp 556:72–80. https://doi.org/10.1016/j.colsurfa.2018.08.009
Zheng Z, Wang Y, Zhao W et al (2017) Adsorptive removal of uranyl ions in aqueous solution using hydrothermal carbon spheres functionalized with 4-aminoacetophenone oxime group. J Radioanal Nucl Chem 312:187–198. https://doi.org/10.1007/s10967-017-5209-y
Elwakeel KZ, El-Bindary AA, Kouta EY, Guibal E (2018) Functionalization of polyacrylonitrile/Na-Y-zeolite composite with amidoxime groups for the sorption of Cu(II), Cd(II) and Pb(II) metal ions. Chem Eng J 332:727–736. https://doi.org/10.1016/j.cej.2017.09.091
Akl ZF, El-Saeed SM, Atta AM (2016) In-situ synthesis of magnetite acrylamide amino-amidoxime nanocomposite adsorbent for highly efficient sorption of U(VI) ions. J Ind Eng Chem 34:105–116. https://doi.org/10.1016/j.jiec.2015.10.042
Yan J, Li Y, Li H et al (2019) Effective removal of ruthenium (III) ions from wastewater by amidoxime modified zeolite X. Microchem J 145:287–294. https://doi.org/10.1016/j.microc.2018.10.047
Abhilash Pandey BD (2013) Microbially assisted leaching of uranium—a review. Miner Process Extr Metall Rev 34:81–113. https://doi.org/10.1080/08827508.2011.635731
Kumar A, Tripathi RM, Rout S et al (2014) Characterization of groundwater composition in Punjab state with special emphasis on uranium content, speciation and mobility. Radiochim Acta 102:239–254. https://doi.org/10.1515/ract-2014-2109
Wei X, Liu Q, Zhang H et al (2017) Efficient removal of uranium(VI) from simulated seawater using amidoximated polyacrylonitrile/FeOOH composites. Dalt Trans 46:15746–15756. https://doi.org/10.1039/c7dt02164j
Kim JS, Zhang L, Keane MA (2001) Removal of iron from aqueous solutions by ion exchange with Na-Y zeolite. Sep Sci Technol 36:1509–1525. https://doi.org/10.1081/SS-100103885
Yang Z, Peng H, Wang W, Liu T (2010) Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J Appl Polym Sci 116:2658–2667. https://doi.org/10.1002/app
Flanigen EM (2001) Chapter 2 Zeolites and molecular sieves: an historical perspective. In: van Bekkum H, Flanigen EM, Jacobs PA, Jansen JC (eds) Introduction to zeolite science and practice. Elsevier, Amsterdam, pp 11–35
Tsantis ST, Zagoraiou E, Savvidou A et al (2016) Binding of oxime group to uranyl ion. Dalt Trans 45:9307–9319. https://doi.org/10.1039/c6dt01293k
Zhao Y, Li J, Zhang S, Wang X (2014) Amidoxime-functionalized magnetic mesoporous silica for selective sorption of U(VI). RSC Adv 4:32710–32717. https://doi.org/10.1039/c4ra05128a
Ji G, Zhu G, Wang X et al (2017) Preparation of amidoxime functionalized SBA-15 with platelet shape and adsorption property of U(VI). Sep Purif Technol 174:455–465. https://doi.org/10.1016/j.seppur.2016.10.048
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dahake, R., Tiwari, P. & Bansiwal, A. Multicycle adsorption and desorption for recovery of U(VI) from aqueous solution using oxime modified zeolite-A. J Radioanal Nucl Chem 327, 133–142 (2021). https://doi.org/10.1007/s10967-020-07482-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07482-1