Abstract
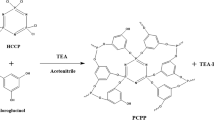
Poly(cyclotriphosphazene-co-polyethyleneimine) microspheres with the solid structure were synthesized by precipitation polymerization method for the separation of highly toxic uranium(VI). The structure and morphology of the prepared microspheres were analyzed via FTIR, SEM–EDS, TEM and BET. The optimal adsorption conditions were also investigated, and the maximum adsorption capacity was 274.2 mg g−1. The experimental results showed that the adsorption kinetics conformed to the pseudo-second-order kinetics and was in good agreement with the Langmuir adsorption isotherm model. Removal of uranium(VI) ions was achieved by the complexation between the amino groups on the adsorbent and uranyl ions.











Similar content being viewed by others
References
Acharya R, Naik B, Parida K (2018) Cr(VI) remediation from aqueous environment through modified-TiO2-mediated photocatalytic reduction. Beilstein J Nanotechnol 9:1448–1470
Pati S, Achary R (2020) An overview on g-C3N4 as a robust photocatalyst towards the sustainable generation of H2 energy. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.04.178
Acharya R, Parida K (2020) A review on TiO2/g-C3N4 visible-light-responsive photocatalysts for sustainable energy generation and environmental remediation. J Environ Chem Eng 8(4):103896
Pravalie R, Bandoc G (2018) Nuclear energy: between global electricity demand, worldwide decarbonisation imperativeness, and planetary environmental implications. J Environ Manag 209:81–92
Feng M-L, Sarma D, Gao Y-J, Qi X-H, Li W-A, Huang X-Y, Kanatzidis MG (2018) Efficient removal of UO22+, Cs+, and Sr2+ ions by radiation-resistant gallium thioantimonates. J Am Chem Soc 140(35):11133–11140
Etale A, Tutu H, Drake DC (2016) The effect of silica and maghemite nanoparticles on remediation of Cu(II)-, Mn(II)- and U(VI)-contaminated water by Acutodesmus sp. J Appl Phycol 28(1):251–260
Zaheri P, Davarkhah R (2017) Rapid removal of uranium from aqueous solution by emulsion liquid membrane containing thenoyltrifluoroacetone. J Environ Chem Eng 5(4):4064–4068
Wu Y, Chen D, Kong L, Tsang DCW, Su M (2019) Rapid and effective removal of uranium(VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. J Hazard Mater 371:397–405
Li X, Qi Y, Yue G, Wu Q, Li Y, Zhang M, Guo X, Li X, Ma L, Li S (2019) Solvent- and catalyst-free synthesis of an azine-linked covalent organic framework and the induced tautomerization in the adsorption of U(VI) and Hg(II). Green Chem 21(3):649–657
Tang M, Chen J, Wang P, Wang C, Ao Y (2018) Highly efficient adsorption of uranium(VI) from aqueous solution by a novel adsorbent: titanium phosphate nanotubes. Environ Sci Nano 5(10):2304–2314
Wang XL, Li Y, Huang J, Zhou YZ, Li BL, Liu DB (2019) Efficiency and mechanism of adsorption of low concentration uranium in water by extracellular polymeric substances. J Environ Radioact 197:81–89
Kanematsu M, Perdrial N, Um W, Chorover J, O’Day PA (2014) Influence of phosphate and silica on U(VI) precipitation from acidic and neutralized wastewaters. Environ Sci Technol 48(11):6097–6106
Zhou L, Wang Y, Zou H, Liang X, Zeng K, Liu Z, Adesina AA (2016) Biosorption characteristics of uranium(VI) and thorium(IV) ions from aqueous solution using CaCl2-modified Giant Kelp biomass. J Radioanal Nucl Chem 307(1):635–644
Khawassek YM, Masoud AM, Taha MH, Hussein AEM (2018) Kinetics and thermodynamics of uranium ion adsorption from waste solution using Amberjet 1200 H as cation exchanger. J Radioanal Nucl Chem 315(3):493–502
Kumar R, Ansari SA, Kandwal P, Mohapatra PK (2020) Pertraction of U(VI) through liquid membranes using monoamides as carrier ligands: experimental and theoretical studies. J Radioanal Nucl Chem 323(2):983–991
Post VEA, Vassolo SI, Tiberghien C, Baranyikwa D, Miburo D (2017) Weathering and evaporation controls on dissolved uranium concentrations in groundwater—a case study from northern Burundi. Sci Total Environ 607:281–293
Saleh TA, Naeemullah Tuzen M, Sari A (2017) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227
Tripathy SP, Subudhi S, Acharya R, Acharya R, Das M, Parida K (2019) Adsorptive removal of Cr(VI) onto UiO-66-NH2 and its determination by radioanalytical techniques. J Radioanal Nucl Chem 322(2):983–992
Niu Y, Lu Z, Li J, Jiang J, Lu J, Wu K (2019) Low-cost synthesis of cellular materials with preformed foam for uranium(VI) adsorption. Mater Lett 247:36–39
Bao SD, Li Y, Fei ZF, Mei H, Zhou YZ, Wang XL, Liu DX (2020) Effective adsorption of uranium(VI) from aqueous solution using ethylene-bridged mesoporous silica functionalized with ureido groups. J Radioanal Nucl Chem 324(1):385–394
El-Sherif RM, Lasheen TA, Jebril EA (2017) Fabrication and characterization of CeO2–TiO2–Fe2O3 magnetic nanoparticles for rapid removal of uranium ions from industrial waste solutions. J Mol Liq 241:260–269
Choi J, Lee JY, Yang J-S (2009) Biosorption of heavy metals and uranium by starfish and Pseudomonas putida. J Hazard Mater 161(1):157–162
Zhu J, Zhang H, Chen R, Liu Q, Liu J, Yu J, Li R, Zhang M, Wang J (2019) An anti-algae adsorbent for uranium extraction: l-Arginine functionalized graphene hydrogel loaded with Ag nanoparticles. J Colloid Interface Sci 543:192–200
Wang XF, Chen YY, Song LP, Fang Z, Zhang J, Shi FN, Lin YW, Sun YK, Zhang YB, Rocha J (2019) Cooperative capture of uranyl ions by a carbonyl-bearing hierarchical-porous Cu-organic framework. Angew Chem Int Ed 58(52):18808–18812
Li P, Chen P, Liu Z, Nie S, Wang X, Wang G, Zhang W, Chen H, Wang L (2020) Highly efficient elimination of uranium from wastewater with facilely synthesized Mg–Fe layered double hydroxides: optimum preparation conditions and adsorption kinetics. Ann Nucl Energy. https://doi.org/10.1016/j.anucene.2019.107140
Liu M, Wang Y, Ma Z, Luo Y (2019) Poly(cyclotriphosphazene-co-phloroglucinol) PCPP as a solid phase extractant for preconcentrative separation of uranium(VI) from aqueous solution. J Radioanal Nucl Chem 319(1):279–288
He X, Schmohl S, Wiemhoefer HD (2019) Comparative study of interfacial behavior for polyphosphazene based polymer electrolytes and LiPF6 in EC/DMC against lithium metal anodes. Polym Test 76:505–512
Liu W, Zhang S, Dar SU, Zhao Y, Akram R, Zhang X, Jin S, Wu Z, Wu D (2018) Polyphosphazene-derived heteroatoms-doped carbon materials for supercapacitor electrodes. Carbon 129:420–427
Huang Z, Gao C, Huang Y, Zhang X, Deng X, Cai Q, Yang X (2018) Injectable polyphosphazene/gelatin hybrid hydrogel for biomedical applications. Mater Des 160:1137–1147
Ni Z, Yu H, Wang L, Shen D, Elshaarani T, Fahad S, Khan A, Haq F, Teng L (2020) Recent research progress on polyphosphazene-based drug delivery systems. J Mater Chem B 8(8):1555–1575
Zhao L, Zhao C, Guo C, Li Y, Li S, Sun L, Li H, Xiang D (2019) Polybenzoxazine resins with polyphosphazene microspheres: synthesis, flame retardancy, mechanisms, and applications. ACS Omega 4(23):20275–20284
Zhao L, Zhao C, Wu M, Li Y, Li H, Xiang D, Guo C (2019) Curing kinetics of phenolphthalein based polyphosphazene towards thermal stability and flame retardancy of polybenzoxazine. RSC Adv 9(54):31583–31593
Dong Y, Zhang Y-X, Xu H-L, Luo T-W, Fu F-Y, Zhu C-J (2016) Proton exchange membrane based on crosslinked sulfonated polyphosphazene containing pendent perfluorosulfonic acid groups with sulfonated poly(ether ether ketone). J Appl Polym Sci 133(23):43492–43501
Fu J, Zhu J, Wang Z, Wang Y, Wang S, Yan R, Xu Q (2019) Highly-efficient and selective adsorption of anionic dyes onto hollow polymer microcapsules having a high surface-density of amino groups: isotherms, kinetics, thermodynamics and mechanism. J Colloid Interface Sci 542:123–135
Liu Y, Dai Y, Yuan D, Wang Y, Zou L (2017) The preparation of PZS-OH/CNT composite and its adsorption of U(VI) in aqueous solutions. J Radioanal Nucl Chem 314(3):1747–1757
Ma Z, Wang Y, Liu M, Luo Y, Xie X, Xiong Z (2019) Highly efficient uranium(VI) removal from aqueous solution using poly(cyclotriphosphazene-co-4,4’-diaminodiphenyl-ether) crosslinked microspheres. J Radioanal Nucl Chem 321(3):1093–1107
Fu J, Chen Z, Wu X, Wang M, Wang X, Zhang J, Zhang J, Xu Q (2015) Hollow poly(cyclotriphosphazene-co-phloroglucinol) microspheres: an effective and selective adsorbent for the removal of cationic dyes from aqueous solution. Chem Eng J 281:42–52
Wang X, Fu J, Chen Z, Li Q, Wu X, Xu Q (2015) Hollow polyphosphazene microspheres with cross-linked chemical structure: synthesis, formation mechanism and applications. RSC Adv 5(43):33720–33728
Zhu L, Xu Y, Yuan W, Xi J, Huang X, Tang X, Zheng S (2006) One-pot synthesis of poly(cyclotriphosphazene-co-4,4’-sulfonyldiphenol) nanotubes via an in situ template approach. Adv Mater 18(22):2997
Chung J, Chun J, Lee J, Lee SH, Lee YJ, Hong SW (2012) Sorption of Pb(II) and Cu(II) onto multi-amine grafted mesoporous silica embedded with nano-magnetite: effects of steric factors. J Hazard Mater 239:183–191
Pan N, Li L, Ding J, Li S, Wang R, Jin Y, Wang X, Xia C (2016) Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). J Hazard Mater 309:107–115
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci 296(2):434–441
El-Maghrabi HH, Younes AA, Salem AR, Rabie K, El-shereafy E (2019) Magnetically modified hydroxyapatite nanoparticles for the removal of uranium(VI): preparation, characterization and adsorption optimization. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.05.096
Li S, Yang P, Liu X, Zhang J, Xie W, Wang C, Liu C, Guo Z (2019) Graphene oxide based dopamine mussel-like cross-linked polyethylene imine nanocomposite coating with enhanced hexavalent uranium adsorption. J Mater Chem A 7(28):16902–16911
Chen YF, Cao LX, Lin H, Dong YB, Huo HX (2013) Adsorption of Cu2+ from simulated acid mine drainage by herb-medicine residues and wheat bran. Z Chin J Nonferrous Met 23(6):1775–1782
Wu X, Lv C, Yu S, Li M, Ye J, Zhang X, Liu Y (2020) Uranium(VI) removal from aqueous solution using iron-carbon micro-electrolysis packing. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2019.116104
Wang B, Li Y, Zheng J, Hu Y, Wang X, Hu B (2020) Efficient removal of U(VI) from aqueous solutions using the magnetic biochar derived from the biomass of a bloom-forming cyanobacterium (Microcystis aeruginosa). Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126898
Wang D, Xu Y, Xiao D, Qiao Q, Yin P, Yang Z, Li J, Winchester W, Wang Z, Hayat T (2019) Ultra-thin iron phosphate nanosheets for high efficient U(VI) adsorption. J Hazard Mater 371:83–93
Pan N, Li L, Ding J, Wang R, Jin Y, Xia C (2017) A Schiff base/quaternary ammonium salt bifunctional graphene oxide as an efficient adsorbent for removal of Th(IV)/U(VI). J Colloid Interface Sci 508:303–312
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Tripathy SP, Acharya R, Das M, Acharya R, Parida K (2020) Adsorptive remediation of Cr(VI) from aqueous solution using cobalt ferrite: kinetics and isotherm studies”. Mater Today Proc 30:289–293
Acharya R, Martha S, Parida KM (2017) Remediation of Cr(VI) using clay minerals, biomasses and industrial wastes as adsorbents. Adv Mater Wast Treat 129–170, ISBN:9781119407768
Liu J-m, Liu T, Wang C-c, Yin X-h, Xiong Z-h (2017) Introduction of amidoxime groups into metal-organic frameworks to synthesize MIL-53(Al)-AO for enhanced U(VI) sorption. J Mol Liq 242:531–536
Simsek S, Senol ZM, Ulusoy HI (2017) Synthesis and characterization of a composite polymeric material including chelating agent for adsorption of uranyl ions. J Hazard Mater 338:437–446
Acknowledgements
The authors gratefully acknowledge support from the National Natural Science Foundation of China (51803088), Scientific Research Fund of Hunan Education Department (17C1360).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, X., Wang, Y., Xiong, Z. et al. Highly efficient removal of uranium(VI) from aqueous solution using poly(cyclotriphosphazene-co-polyethyleneimine) microspheres. J Radioanal Nucl Chem 326, 1867–1877 (2020). https://doi.org/10.1007/s10967-020-07455-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07455-4