Abstract
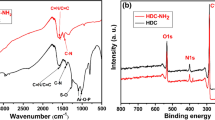
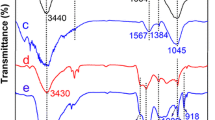
In this study, hydrothermal carbon doped with phosphorus using phytic acid (PA) and Sodium tripolyphosphate as functional monomers was successfully synthesized by the hydrothermal route combined with microwave-assisted hydrothermal carbon as matrix material. The uranium(VI) adsorption capacity by phytic acid functionalized microwave-assisted hydrothermal carbon (MHC-PA) was up to 382.2 mg/g at pH of 6.0 and the temperature of 298.15 K. The adsorption mechanism of uranium(VI) onto the MHC-PA was mainly the chelation of P=O, P–OH functional group and uranium(VI), which improves the selective adsorption performance of MHC-PA for uranium(VI).


















Similar content being viewed by others
References
Anirudhan TS, Bringle CD, Rijith S (2010) Removal of uranium(VI) from aqueous solutionsand nuclear industry effluents using humic acid-immobilized zirconium-pillared clay. J Environ Radioact 101:267–276
Zhang CL, Li X, Chen ZS, Wen T, Huang SY, Hayat T, Alsaedi A, Wang XK (2018) Synthesis of ordered mesoporous carbonaceous materials and their highly efficient capture of uranium from solutions. Sci China Chem 61:281–293
Aly Z, Luca V (2013) Uranium extraction from aqueous solution using dried and pyrolyzed tea and coffee wastes. J Radioanal Nucl Chem 295:889–900
Torkabad MG, Keshtkar AR, Safdari SJ (2017) Comparison of polyethersulfone and polyamide nanofiltration membranes for uranium removal from aqueous solution. Prog Nucl Energy 94:93–100
Duan SX, Liu X, Wang YN, Shao DD, Alharbi NS, Alsaedi A, Li JX (2016) Highly efficient entrapment of U(VI) by using porousmagnetic Ni0.6Fe2.4O4 micro-particles asthe adsorbent. J Hazard Mater 65:367–377
Mustafa T, Tawfik AS, Ahmet S, Naeemullah (2020) Interfacial polymerization of trimesoyl chloride with melamine and palygorskite for efficient uranium ions ultra-removal. Chem Eng Res Des 159:353–361
Esra B, Ahmet S, Mustafa T (2018) Effective uranium biosorption by macrofungus (Russula sanguinea) from aqueous solution: equilibrium, thermodynamic and kinetic studies. J Radioanal Nucl Chem 317:1387–1397
Tawfik AS, Naeemullah Mustafa T, Ahmet S (2017) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227
Niu YH, Lu ZY, Li J, Jiang J, Lu J, Wu KP (2019) Low-cost synthesis of cellular materials with preformed foam for uranium(VI) adsorption. Mater Lett 247:36–39
Lai ZJ, Zhang ZB, Cao XH, Dai Y, Hua R, Le ZG, Luo MB, Liu YH (2016) Synthesis of novel functional hydrothermal carbon spheres for removal of uranium from aqueous solution. J Radioanal Nucl Chem 310:1335–1344
Zheng ZY, Wang YQ, Zhao WW, Xiong GX, Cao XH, Dai Y, Le ZG, Yu SL, Zhang ZB, Liu YH (2017) Adsorptive removal of uranyl ions in aqueous solutionusing hydrothermal carbon spheres functionalizedwith 4-aminoacetophenone oxime group. J Radioanal Nucl Chem 312:187–198
Zhang ZB, Liu YH, Cao XH, Liang P (2013) Sorption study of uranium on carbon spheres hydrothermal synthesized with glucose from aqueous solution. J Radioanal Nucl Chem 295:1775–1782
Adolfsson KH, Lin CF, Hakkarainen M (2018) Microwave assisted hydrothermal carbonization and solid state postmodification of carbonized polypropylene. ACS Sustain Chem Eng 6:11105–11114
Alexandre L, Hibiki U, Ryo T, Polina M, Naritaka K, Ryuzo K, Seiichiro N (2017) Monitoring and extraction of uranium in polluted acid mine drainage by super-paramagnetic nanoparticles coated with carbon nanodots. J Radioanal Nucl Chem 314:1149–1159
Xu DD, Xu TY, Guo XJ, Liu Q, Liu JY, Lv WZ, Jing XY, Zhang HS, Wang J (2017) Effect of the synthesis method on the performance of Fe3O4–inositol hexaphosphate as a drug delivery vehicle for combination therapeutics with doxorubicin. New J Chem 41:5305–5312
Lei H, Pan N, Wang XQ, Zou H (2018) Facile synthesis of phytic acid impregnated polyaniline for enhanced U(VI) adsorption. J Chem Eng Data 63:3989–3997
Wang J, Wen X, Yang F, Cao ZF, Wang S, Zhong H (2018) Preparation of a novel two-dimensional carbon material and enhancing Cu(II) ions removal by phytic acid. Environ Earth Sci 77:472
Liu SJ, Ma JG, Zhang WQ, Luo F, Luo MB, Li FQ, Wu LP (2015) Three-dimensional graphene oxide/phytic acid composite for uranium(VI) sorption. J Radioanal Nucl Chem 306:507–514
Gurses MS, Erkey C, Kizilel S, Uzun A (2018) Characterization of sodiumtripolyphosphate and sodium citrate dehydrate residues on surfaces. Talanta 176:8–16
Chu HH, Lin XY, Li MS, Liang LL, Zhou J, Shang R, Luo XG (2019) Rapid synthesis of carbon materials by microwave-assisted hydrothermal method at low temperature and its adsorption properties for uranium(VI). J Radioanal Nucl Chem 321:629–646
Zhao WH, Lin XY, Cai HM, Mu T, Luo XG (2017) Preparation of mesoporous carbon from sodium lignosulfonate by hydrothermal and template method and its adsorption of uranium(VI). Ind Eng Chem Res 56:12745–12754
Chen Z, Ma LJ, Li SQ, Geng JX, Song Q, Liu J, Wang CL, Wang H, Li J, Qin Z, Li SJ (2011) Simple approach to carboxyl-rich materials through low-temperature heat treatment of hydrothermal carbon in air. Appl Surf Sci 257:8686–8691
Fanning PE, Vannice MA (1993) A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 31:721–730
Pan N, Jin YD, Wang XQ, Hu XP, Chi FT, Zou H, Xia CQ (2019) A self-assembled supramolecular material containing phosphoric acid for ultrafast and efficient capture of uranium from acidic solutions. ACS Sustain Chem Eng 7:950–960
Li HY, Liu L, Zhang ZW, Wang SS, Yu Y, Liu L, Wu Y (2017) Phytic acid-assisted electrochemically synthesize three-dimensional O, P-functionalized graphene monolith with high capacitive performance. Nanoscale 9:12601–12608
Ehsanjoo M, Mohammadi S, Chaibakhsh N (2019) Long-term corrosion resistance of zinc-rich paint using functionalised multi-layer graphene-tripolyphosphate: in situ creation of zinc phosphate as corrosion inhibitor. Corros Eng Sci Technol 54:698–714
Yu J, Luo XG, Liu B, Zhou J, Feng J, Zhu WK, Wang SL, Zhang YD, Lin XY, Chen P (2018) Bayberry Tannin immobilized bovine serum albumin nanospheres: characterization, irradiation stability and selective removal uranyl ion from radioactive wastewater. J Mater Chem A 6:15359–15370
Cai YW, Wu CF, Liu ZY, Zhang LJ, Chen LH, Wang JQ, Wang XK, Yang ST, Wang S (2017) Fabrication of phosphorylated graphene oxide-chitosan composite for highly effective and selective capture of U(VI). Environ-Sci Nano 4:1876–1886
Liao Y, Wang M, Chen DJ (2019) Electrosorption of uranium(VI) by highly porous phosphate-functionalized graphene hydrogel. Appl Surf Sci 484:83–96
Ding CC, Cheng WC, Nie XQ, Yi FC (2017) Synergistic mechanism of U(VI) sequestration by magnetite-graphene oxide composites: evidence from spectroscopic and theoretical calculation. Chem Eng J 324:113–121
Zhang ZB, Dong ZM, Dai Y, Xiao SJ, Cao XH, Liu YH, Guo WH, Luo MB, Le ZG (2016) Amidoxime-functionalized hydrothermal carbon material for uranium removal from aqueous solution. RSC Adv 6:102462–102471
Monier M, Elsayed NH (2014) Selective extraction of uranyl ions using ion-imprinted chelating microspheres. J Colloid Interface Sci 423:113–122
Wu LP, Lin XY, Du XC, Luo XG (2016) Biosorption of uranium(VI) from aqueous solution using microsphere adsorbents of carboxymethyl cellulose loaded with aluminum(III). J Radioanal Nucl Chem 310:611–622
Zhang YH, Lin XY, Zhou QS, Luo XG (2016) Fluoride adsorption from aqueous solution by magnetic core-shell Fe3O4@alginate-La particles fabricated via electro-coextrusion. Appl Surf Sci 389:34–45
Sivakami MS, Gomathi T, Venkatesan J, Jeong HS, Kim SK, Sudha PN (2013) Preparation and characterization of nano chitosan for treatment wastewaters. Int J Biol Macromol 57:204–212
Liu YH, Wang YQ, Zhang ZB, Cao XH, Nie WB, Li Q, Hua R (2013) Removal of uranium from aqueous solution by a low cost and high-efficient adsorbent. Appl Surf Sci 273:68–74
Anirudhan TS, Lekshmi GS, Shainy F (2018) Synthesis and characterization of amidoxime modified chitosan/bentonite composite for the adsorptive removal and recovery of uranium from seawater. J Colloid Interface Sci 534:248–261
Nilchi A, Dehaghan TS, Garmarodi SR (2013) Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxidenanoparticles. Desalination 321:67–71
Sureshkumar MK, Das D, Mallia MB, Gupta PC (2010) Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate(CTPP)beads. J Hazard Mater 184:65–72
Chen JF, Zhang WY, Li X (2016) Adsorption of Cu(II) ion from aqueous solutions on hydrogel prepared from Konjac glucomannan. Polym Bull 73:1965–1984
Anirudhan TS, Nima J, Divya PL (2015) Adsorption and separation behavior of uranium(VI) by 4-vinylpyridine-grafted-vinyltriethoxysilane-cellulose ion imprinted polymer. J Environ Chem Eng 3:1267–1276
Bayramoglu G, Arica MY (2016) MCM-41 silica particles grafted with polyacrylonitrile: modification into amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous Mesoporous Mater 226:117–124
Li J, Zhang SW, Chen CL, Zhao GX, Yang X, Li JX, Wang XK (2012) Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl Mater Interfaces 4:4991–5000
Zhou QS, Lin XY, Qian J, Wang J, Luo XG (2015) Porous zirconium alginate beads adsorbent forfluoride adsorption from aqueous solutions. RSC Adv 5:2100–2112
Geng JX, Ma LJ, Wang H, Liu J, Bai CY, Song Q, Li J, Hou M, Li SJ (2012) Amidoxime-grafted hydrothermal carbon microspheres for highly selective separation of uranium. J Nanosci Nanotechnol 12:7354–7363
Wang Y, Gu ZX, Yang JJ, Liao JL, Yang YY, Liu N, Tang J (2014) Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(VI). Appl Surf Sci 320:10–20
Acknowledgements
The authors would like to thank the technology support of Engineering Research Center of Biomass Materials, Ministry of Education, Southwest University of Science and Technology. This work was sponsored by Longshan academic talent research support plan of Southwest University of Science and Technology (18LZX315) and Military special research projects on decommissioning of nuclear facilities and radioactive waste treatment (14zg6101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, L., Lin, X., Li, M. et al. Microwave-assisted hydrothermal synthesis of carbon doped with phosphorus for uranium(VI) adsorption. J Radioanal Nucl Chem 327, 73–89 (2021). https://doi.org/10.1007/s10967-020-07453-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07453-6