Abstract
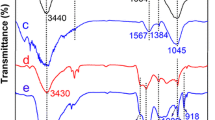
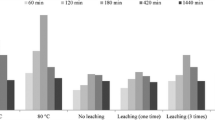
Manganese ferrite/porous graphite carbon nitride composites (MnFe2O4/porous g-C3N4[PCN]) were synthesized by template-assisted thermal polymerization and hydrothermal method and applied for high adsorption of U(VI) from aqueous solution. Results indicated that the maximum adsorption capacity of MnFe2O4/PCN (MPCN) was 367.9 mg g−1 under optimum conditions (pH = 5, t = 15 min, and T = 308 K), which was higher than that of g-C3N4 (185.1 mg g−1). Adsorption was well fitted with Langmuir isotherm model and pseudo-second-order kinetic equation. Thermodynamic analysis showed that adsorption is an endothermic, spontaneous process. X-ray photoelectron spectra revealed that amino and metal oxygen groups are involved in the complexing adsorption of uranium. Adsorption efficiency remained 92.5% after five adsorption–desorption cycles. The results of this paper indicated that MnFe2O4/PCN composites can be efficiently used as an ideal material for U(VI) adsorption.











Similar content being viewed by others

References
Gu P, Zhang S, Li X et al (2018) Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ Pollut 240:493–505
Kou Y, Zhang L, Liu B et al (2019) Phosphonate modified MoS2 composite material for effective adsorption of uranium(VI) in aqueous solution. J Radioanal Nucl Chem 323(1):641–649
Jiaqi Z, Yimin D, Danyang L et al (2019) Synthesis of carboxyl-functionalized magnetic nanoparticle for the removal of methylene blue. Colloids Surf A 572(58–66):22
Bao S, Yang W, Wang Y et al (2020) One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solution. J Hazard Mater 381:120914
Xu W, Song Y, Dai K et al (2018) Novel ternary nanohybrids of tetraethylenepentamine and graphene oxide decorated with MnFe2O4 magnetic nanoparticles for the adsorption of Pb(II). J Hazard Mater 358:337–345
Fang Q, Duan S, Zhang J et al (2017) Dual shelled Fe3O4/polydopamine hollow microspheres as an effective Eu(III) adsorbent. J Mater Chem A 5(6):2947–2958
Fang JUN, Gu Z, Gang D et al (2007) Cr(VI) removal from aqueous solution by activated carbon coated with quaternized poly (4-vinylpyridine). Environ Sci Technol 41(13):4748–4753
Beygli RA, Mohaghegh N, Rahimi E (2019) Metal ion adsorption from wastewater by g-C3N4 modified with hydroxyapatite: a case study from Sarcheshmeh acid mine drainage. Res Chem Intermed 45(4):2255–2268
Xiong C, Wang S, Sun W et al (2019) Selective adsorption of Pb(II) from aqueous solution using nanosilica functionalized with diethanolamine: equilibrium, kinetic and thermodynamic. Microchem J 146:270–278
Gao H, Sun Y, Zhou J et al (2013) Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl Mater Interfaces 5(2):425–432
Zhu K, Chen C, Xu H et al (2017) Cr(VI) reduction and immobilization by core-double-shell structured magnetic polydopamine @ zeolitic idazolate frameworks-8 microspheres. ACS Sustain Chem Eng 5(8):6795–6802
Liu H, Zhou Y, Yang Y et al (2019) Synthesis of polyethylenimine/graphene oxide for the adsorption of U(VI) from aqueous solution. Appl Surf Sci 471:88–95
Zhang Y, Pan Q, Chai G et al (2013) Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci Rep 3:1943
Chen Y, Lin B, Wang H et al (2016) Surface modification of g-C3N4 by hydrazine: simple way for noble-metal free hydrogen evolution catalysts. Chem Eng J 286:339–346
Panneri S, Ganguly P, Nair BN et al (2017) Role of precursors on the photophysical properties of carbon nitride and its application for antibiotic degradation. Environ Sci Pollut Res 24(9):8609–8618
Gong Y, Li M, Li H et al (2015) Graphitic carbon nitride polymers: promising catalysts or catalyst supports for heterogeneous oxidation and hydrogenation. Green Chem 17(2):715–736
Wang X, Maeda K, Thomas A et al (2009) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8(1):76–80
Song B, Zeng Z, Zeng G et al (2019) Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: a review of strategy, synthesis, and applications. Adv Colloid Interface Sci 272:101999
Liu Y, Wu YH, Pang HW et al (2019) Study on the removal of water pollutants by graphite phase carbon nitride materials. Progr Chem 31(06):831–846
Chen H, Yan T, Jiang F (2014) Adsorption of Cr(VI) from aqueous solution on mesoporous carbon nitride. J Taiwan Inst Chem Eng 45(4):1842–1849
Kafshgari LA, Ghorbani M, Azizi A (2017) Fabrication and investigation of MnFe2O4/MWCNTs nanocomposite by hydrothermal technique and adsorption of cationic and anionic dyes. Appl Surf Sci 419:70–83
Yao Y, Cai Y, Lu F et al (2014) Magnetic recoverable MnFe2O4 and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate activation for efficient degradation of aqueous organic pollutants. J Hazard Mater 270:61–70
Chen YX, Li RC, Zhang YH, Chen XG, Ye Y (2017) Adsorption of methylene blue by halloysite/MnFe2O4 nanocomposites. J Nanosci Nanotechnol 17(9):6489–6496
Li N, Fu F, Lu J, Ding Z, Tang B, Pang J (2017) Facile preparation of magnetic mesoporous MnFe2O4@ SiO2-CTAB composites for Cr(VI) adsorption and reduction. Environ Pollut 220:1376–1385
Podder MS, Majumder CB (2016) Studies on the removal of As(III) and As(V) through their adsorption onto granular activated carbon/MnFe2O4 composite: isotherm studies and error analysis. Compos Interfaces 23(4):327–372
Liu J, Chen Z, Yu K, LiuY GY, Xie S (2019) Polyaniline/oxidation etching graphitic carbon nitride composites for U(VI) removal from aqueous solutions. J Radioanal Nucl Chem 321(3):1005–1017
Liu H, Dai P, Zhang J et al (2013) Preparation and evaluation of activated carbons from lotus stalk with trimethyl phosphate and tributyl phosphate activation for lead removal. Chem Eng J 228:425–434
Liu X, Liu Y, Zhang W et al (2020) In situ self-assembly of 3D hierarchical 2D/2D CdS/g-C3N4 hereojunction with excellent photocatalytic performance. Mater Sci Semicond Process 105:104734
Vignesh K, Suganthi A, Min BK et al (2014) Photocatalytic activity of magnetically recoverable MnFe2O4/g-C3N4/TiO2 nanocomposite under simulated solar light irradiation. J Mol Catal A Chem 395:373–383
Ou B, Wang J, Wu Y et al (2020) Efficient removal of Cr(VI) by magnetic and recyclable calcined CoFe-LDH/g-C3N4 via the synergy of adsorption and photocatalysis under visible light. Chem Eng J 380:122600
Zhang S, Li J, Zeng M et al (2013) In situ synthesis of water-soluble magnetic graphitic carbon nitride photocatalyst and its synergistic catalytic performance. ACS Appl Mater Interfaces 5(23):12735–12743
Gebreslassie G, Bharali P, Chandra U et al (2019) Novel g-C3N4/graphene/NiFe2O4 nanocomposites as magnetically separable visible light driven photocatalysts. J Photochem Photobiol A 382:111960
Palanivel B, Maiyalagan T, Jayarman V et al (2019) Rational design of ZnFe2O4/g-C3N4 nanocomposite for enhanced photo-Fenton reaction and supercapacitor performance. Appl Surf Sci 498:143807
Abdul Rahman AJ, Fiona HNF, Mohd Nasir MH et al (2019) S-quinolin-2-yl-methyldithiocarbazate-based magnetic adsorbent for magnetic solid-phase extraction of heavy metals from water samples. Int J Environ Anal Chem 1–18
Jung C, Park J, Lim KH et al (2013) Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochar. J Hazard Mater 263:702–710
Pang C, Liu Y, Cao X et al (2010) Adsorptive removal of uranium from aqueous solution using chitosan-coated attapulgite. J Radioanal Nucl Chem 286(1):185–193
Zhang X, Xue Y, Gao J et al (2020) Comparison of adsorption mechanisms for cadmium removal by modified zeolites and sands coated with Zn-layered double hydroxides. Chem Eng J 380:122578
Enniya I, Rghioui L, Jourani A (2018) Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain Chem Pharm 7:9–16
Zhao G, Wen T, Yang X, Yang S, Liao J, Hu J, Shao D, Wang X (2012) Preconcentration of U(VI) ions on few-layered graphene oxide nanoshe from aqueous solutions. Dalton Trans 41(20):6182–6188
Shehzad H, Zhou L, Li Z, Chen Q, Wang Y, Liu Z, Adesina AA (2018) Effective adsorption of U(VI) from aqueous solution using magnetic chitosan nanoparticles grafted with maleic anhydride: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 315(2):195–206
Jin J, Li S, Peng X, Liu W, Zhang C, Yang Y, Wang X (2018) HNO3 modified biochars for uranium(VI) removal from aqueous solution. Bioresour Technol 256:247–253
Liu H, Xie S, Liao J, Yan T, Liu Y, Tang X (2018) Novel graphene oxide/bentonite composite for uranium(VI) adsorption from aqueous solution. J Radioanal Nucl Chem 317(3):1349–1360
Li M, Liu H, Chen T, Dong C, Sun Y (2019) Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci Total Environ 651:1020–1028
Yu B, Ye G, Chen J, Ma S (2019) Membrane-supported 1D MOF hollow superstructure array prepared by polydopamine-regulated contra-diffusion synthesis for uranium entrapment. Environ Pollut 253:39–48
Sun Y, Yang S, Sheng G et al (2011) Comparison of U(VI) removal from contaminated groundwater by nanoporous alumina and non-nanoporous alumina. Sep Purif Technol 83:196–203
Yu S, Wang J, Song S, Sun K, Li J, Wang X, Wang X (2017) One-pot synthesis of graphene oxide and Ni–Al layered double hydroxides nanocomposites for the efficient removal of U(VI) from wastewater. Sci China Chem 60(3):415–422
Liu X, Cheng C, Xiao C, Shao D, Xu Z, Wang J, Wang W (2017) Polyaniline (PANI) modified bentonite by plasma technique for U(VI) removal from aqueous solution. Appl Surf Sci 411:331–337
Yan T, Luo X, Zou Z et al (2017) Adsorption of uranium(VI) from a simulated saline solution by alkali-activated leather waste. Ind Eng Chem Res 56(12):3251–3258
Abdi S, Nasiri M, Mesbahi A et al (2017) Investigation of uranium(VI) adsorption by polypyrrole. J Hazard Mater 332:132–139
Zhang Z, Nie W, Li Q et al (2013) Removal of uranium(VI) from aqueous solutions by carboxyl-rich hydrothermal carbon spheres through low-temperature heat treatment in air. J Radioanal Nucl Chem 298(1):361–368
Ren X, Wang S, Yang S et al (2009) Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX-80 bentonite. J Radioanal Nucl Chem 283(1):253–259
Liu J, Zhao C, Tu H et al (2017) U(VI) adsorption onto cetyltrimethylammonium bromide modified bentonite in the presence of U(VI)-CO3 complexes. Appl Clay Sci 135:64–74
Liatsou I, Pashalidis I, Dosche C (2020) Cu(II) adsorption on 2-thiouracil-modified Luffa Cylindrica biochar fibres from artificial and real samples, and competition reactions with U(VI). J Hazard Mater 383:120950
Azoulay A, Barrio J, Shalom M (2019) Modifying crystallinity morphology, and photophysical properties of carbon nitride by using crystals as reactants. Isr J Chem 59:1–7
Chaudhary M, Singh L, Rekha P et al (2019) Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine. Chem Eng J 378:122236
Li X, Xing J, Zhang C et al (2018) Adsorption of lead on sulfur-doped graphitic carbon nitride nanosheets: experimental and theoretical calculation study. ACS Sustain Chem Eng 6(8):10606–10615
Yao Y, Cai Y, Lu F et al (2014) Magnetic ZnFe2O4/g-C3N4 hybrid for photocatalytic degradation of aqueous organic pollutants by visible light. Ind Eng Chem Res 53(44):17294–17302
Hong D, Yamada Y, Nagatomi T et al (2012) Catalysis of nickel ferrite for photocatalytic water oxidation using [Ru (bpy) 3]2+ and S2O82−. J Am Chem Soc 134(48):19572–19575
Fu Y, Xiong P, Chen H et al (2012) High photocatalytic activity of magnetically separable manganese ferrite–graphene heteroarchitectures. Ind Eng Chem Res 51(2):725–731
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant Numbers NO51904155].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, Y., He, Z., Wu, J. et al. Manganese ferrite/porous graphite carbon nitride composites for U(VI) adsorption from aqueous solutions. J Radioanal Nucl Chem 326, 157–171 (2020). https://doi.org/10.1007/s10967-020-07281-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07281-8