Abstract
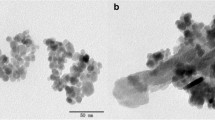
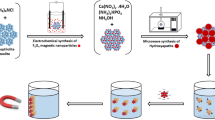
In this work, the magnetic hydroxyapatite composite (Fe3O4@HAP) was prepared and used to remove of U(VI). The characterizations of transmission electron microscope and vibrating sample magnetometer indicated that the average size and saturation magnetizations (Ms) of Fe3O4@HAP was about 360 nm and 20.89 emu g−1, respectively. The results of solid-phase extraction indicated that at pH 5.0, the removal efficiency of Fe3O4@HAP was more than 95% within 20 min. The maximum adsorption capacity (qm) of Fe3O4@HAP was 789.58 mg g−1. Therefore, Fe3O4@HAP may be a rapid and highly efficient magnetic adsorbent to remove of U(VI) from water.








Similar content being viewed by others
References
Wang YQ, Zhen Z, Zhao Y, Huang J, Zhang Z, Cao X, Dai Y, Hua R, Liu Y (2018) Adsorption of U(VI) on montmorillonite pillared with hydroxy-aluminum. J Radioanal Nucl Chem 317(1):69–80
Wang L, Li Z, Wu Q, Huang Z, Yuan L, Chai Z, Shi W (2020) Layered structure-based materials: challenges and opportunities for radionuclide sequestration. Environ Sci Nano 7:724–752
Ansoborlo E, Lebaron-Jacobs L, Prat O (2015) Uranium in drinking-water: a unique case of guideline value increases and discrepancies between chemical and radiochemical guidelines. Environ Int 77:1–4
Wu Y, Pang H, Liu Y, Wang X, Yu S, Fu D, Chen J, Wang X (2019) Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut 246:608–620
Mehta D, Mazumdar S, Singh S (2015) Magnetic adsorbents for the treatment of water/wastewater—a review. J Water Process Eng 7:244–265
Zhang Z, Dong Z, Wang X, Dai Y, Cao X, Wang Y, Hua R, Feng H, Chen J, Liu Y (2019) Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U(VI): batch and fixed-bed column studies. Chem Eng J 370:1376–1387
Han X, Wang Y, Cao X, Dai Y, Liu Y, Dong Z, Zhang Z, Liu Y (2019) Adsorptive performance of ship-type nano-cage polyoxometalates for U(VI) in aqueous solution. Appl Surf Sci 484:1035–1040
Kulkarni P, Mukhopadhyay S, Bellary M, Ghosh S (2002) Studies on membrane stability and recovery of uranium(VI) from aqueous solutions using a liquid emulsion membrane process. Hydrometallurgy 64(1):49–58
Hokkanen S, Bhatnagar A, Repo E, Lou S, Sillanpää M (2016) Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution. Chem Eng J 283:445–452
Wang Y, Liu YX, Lu H, Yang R, Yang S (2018) Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions. J Solid State Chem 261:53–61
Kong L, Ruan Y, Zheng Q, Su M, Diao Z, Chen D, Hou LA, Chang X, Shih K (2020) Uranium extraction using hydroxyapatite recovered from phosphorus containing wastewater. J Hazard Mater 382:120784
Skwarek E, Gładysz-Płaska A, Choromańska J, Broda E (2019) Adsorption of uranium ions on nano-hydroxyapatite and modified by Ca and Ag ions. Adsorption 25(3):639–647
Kim H, Um W, Kim W-S, Chang S (2017) Synthesis of tributyl phosphate-coated hydroxyapatite for selective uranium removal. Ind Eng Chem Res 56(12):3399–3406
Yang D, Wang X, Song G, Zhao G, Chen Z, Yu S, Gu P, Wang H, Wang X (2017) One-pot synthesis of arginine modified hydroxyapatite carbon microsphere composites for efficient removal of U(VI) from aqueous solutions. Sci Bull 62(23):1609–1618
Srilakshmi C, Saraf R (2016) Ag-doped hydroxyapatite as efficient adsorbent for removal of Congo red dye from aqueous solution: synthesis, kinetic and equilibrium adsorption isotherm analysis. Microporous Mesoporous Mat 219:134–144
Ciobanu G, Barna S, Harja M (2016) Kinetic and equilibrium studies on adsorption of Reactive Blue 19 dye from aqueous solutions by nanohydroxyapatite adsorbent. Arch Environ Prot 42(2):3–11
Wu Y, Chen D, Kong L, Tsang DC, Su M (2019) Rapid and effective removal of uranium(VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. J Hazard Mater 371:397–405
Wang Y, Hu L, Zhang G, Yan T, Yan L, Wei Q, Du B (2017) Removal of Pb(II) and methylene blue from aqueous solution by magnetic hydroxyapatite-immobilized oxidized multi-walled carbon nanotubes. J Colloid Interface Sci 494:380–388
Hemmati M, Rajabi M, Asghari A (2018) Magnetic nanoparticle based solid-phase extraction of heavy metal ions: a review on recent advances. Microchim Acta 185(3):160
Wang C, Feng C, Gao Y, Ma X, Wu Q, Wang Z (2011) Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem Eng J 173(1):92–97
Gong X, Liao G, Xuan S (2012) Full-field deformation of magnetorheological elastomer under uniform magnetic field. Appl Phys Lett 100(21):211909
Zhuang F, Tan R, Shen W, Zhang X, Xu W, Song W (2015) Monodisperse magnetic hydroxyapatite/Fe3O4 microspheres for removal of lead(II) from aqueous solution. J Alloys Compd 637:531–537
Deng H, Li X, Peng Q, Wang X, Chen J, Li Y (2005) Monodisperse magnetic single-crystal ferrite microspheres. Angew Chem Int Ed 117(18):2842–2845
Chen H, Wang Y, Zhao W, Xiong G, Cao X, Dai Y, Le Z, Zhang Z, Liu Y (2017) Phosphorylation of graphehe oxide to improve adsorption of U(VI) from aquaeous solutions. J Radioanal Nucl Chem 313(1):175–189
Fannin P, Marin C, Malaescu I, Stefu N (2007) An investigation of the microscopic and macroscopic properties of magnetic fluids. Phys B 388(1–2):87–92
Aviles MO, Chen H, Ebner AD, Rosengart AJ, Kaminski MD, Ritter JA (2007) In vitro study of ferromagnetic stents for implant assisted-magnetic drug targeting. J Magn Magn Mater 311(1):306–311
Veisi H, Safarimehr P, Hemmati S (2018) Oxo-vanadium immobilized on polydopamine coated-magnetic nanoparticles (Fe3O4): a heterogeneous nanocatalyst for selective oxidation of sulfides and benzylic alcohols with H2O2. J Taiwan Inst Chem E 88:8–17
Shinde SK, Dubal DP, Ghodake GS, Fulari VJ (2015) Hierarchical 3D-flower-like CuO nanostructure on copper foil for supercapacitors. RSC Adv 5(6):4443–4447
Zhang Z, Liu J, Cao X, Luo X, Hua R, Liu Y, Yu X, He L, Liu Y (2015) Comparison of U(VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U(VI)–CO3/Ca–U(VI)–CO3 complexes. J Hazard Mater 300:633–642
Zhang Z, Dong Z, Dai Y, Xiao S, Cao X, Liu Y, Guo W, Luo M, Le Z (2016) Amidoxime-functionalized hydrothermal carbon materials for uranium removal from aqueous solution. RSC Adv 6(104):102462–102471
Gao B, Li Y, Chen Z (2009) Adsorption behaviour of functional grafting particles based on polyethyleneimine for chromate anions. Chem Eng J 150(2–3):337–343
Camacho LM, Deng S, Parra RR (2010) Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater 175(1–3):393–398
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Xu J, Chen M, Zhang C (2013) Adsorption of uranium(VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan. J Radioanal Nucl Chem 298(2):1375–1383
Zou W, Lei Z (2012) Removal of uranium(VI) from aqueous solution using citric acid modified pine sawdust: batch and column studies. J Radioanal Nucl Chem 292(2):585–595
Chen R, Chai L, Li Q, Shi Y (2013) Preparation and characterization of magnetic Fe3O4/CNT nanoparticles by RPO method to enhance the efficient removal of Cr(VI). Environ Sci Pollut Res 20(10):7175–7185
Zou WH, Zhao L, Zhu L (2012) Efficient uranium(VI) biosorption on grapefruit peel: kinetic study and thermodynamic parameters. J Radioanal Nucl Chem 292(3):1303–1315
Ho Y-S (2006) Review of second-order models for adsorption systems. J Hazard Mater 136(3):681–689
Wu FC, Tseng R-L, Huang S-C, Juang R-S (2009) Characteristics of pseudo-second-order kinetic model for liquid-phase adsorption: a mini-review. Chem Eng J 151(1–3):1–9
Alkan M, Demirbaş Ö, Doğan M (2007) Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mat 101(3):388–396
Qiu H, Lv L, Pan B-c, Zhang Q-j, Zhang W-m, Zhang Q-x (2009) Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 10(5):716–724
Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98(15):2897–2904
Fuller C, Bargar J, Davis J, Piana M (2002) Mechanisms of uranium interactions with hydroxyapatite: implications for groundwater remediation. Environ Sci Technol 36(2):158–165
Chang MC, Tanaka J (2002) FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 23(24):4811–4818
Vidya K, Dapurkar S, Selvam P, Badamali S, Gupta N (2001) The entrapment of UO22+ in mesoporous MCM-41 and MCM-48 molecular sieves. Microporous Mesoporous Mat 50(2–3):173–179
Sneha M, Sundaram NM (2015) Preparation and characterization of an iron oxide-hydroxyapatite nanocomposite for potential bone cancer therapy. Int J Nanomed. https://doi.org/10.2147/IJN.S79985
Zheng Z, Wan J, Song X, Tokunaga TK (2006) Sodium meta-autunite colloids: synthesis, characterization, and stability. Colloid Surf A 274(1–3):48–55
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21906017, 21866004, 21866003), the Science and Technology Support Program of Jiangxi Province (Grant No. 2018ACB21007), the Jiangxi Program of Academic and Technical Leaders of Major Disciplines (Grant No. 20182BCB22011), the Project of the Jiangxi Provincial Department of Education (Grant Nos. GJJ160550, GJJ160577, GJJ180385, GJJ180400). The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, D., Dai, Y., Zhang, Z. et al. Magnetic solid-phase extraction of U(VI) in aqueous solution by Fe3O4@hydroxyapatite. J Radioanal Nucl Chem 324, 1329–1337 (2020). https://doi.org/10.1007/s10967-020-07148-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07148-y