Abstract
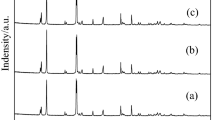
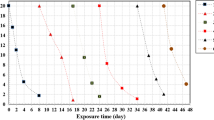
As jarosite and oxalic acid are ubiquitous in nature, we investigated a remediation technology for uranium-contaminated site by photoinduced reduction coupled with adsorption of jarosite. Jarosite exhibited excellent adsorption properties for uranium, with a highest adsorption capacity 453.98 mg g−1. With the presence of oxalic acid, U(VI) was removed at a reduction ratio of 30.71% under irradiation. It may be that oxalic acid promoted the reduction of Fe(III) to Fe(II) in jarosite, and then Fe(II) reduced U(VI) to U(IV). The results confirmed the applicability of the remediation by adsorption using jarosite and light-induced reduction.









Similar content being viewed by others
References
Costa MR, Pereira AJSC, Neves LJPF, Ferreira A (2017) Potential human health impact of groundwater in non-exploited uranium ores: the case of horta da Vilariça (NE Portugal). J Geochem Explor 183:191–196
Ma W, Gao B, Guo Y, Sun Z, Zhang Y, Chen G, Zhu X, Zhang C (2020) Occurrence and distribution of uranium in a hydrological cycle around a uranium mill tailings Pond, Southern China. Int J Environ Res Public Health 17(3):773
Abdelouas A (2006) Uranium mill tailings: geochemistry, mineralogy, and environmental impact. Elements 2(6):335–341
Domingo JL (2001) Reproductive and developmental toxicity of natural and depleted uranium: a review. Reprod Toxicol 15(6):603–609
Radespiel-Tröger M, Meyer M (2013) Association between drinking water uranium content and cancer risk in Bavaria. Germany Int Arch Occup Environ Health 86(7):767–776
Wang S, Ran Y, Lu B, Li J, Kuang H, Gong L, Hao Y (2019) A review of uranium-induced reproductive toxicity. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01920-2
Bjørklund G, Semenova Y, Pivina L, Dadar M, Rahman MM, Aaseth J, Chirumbolo S (2020) Uranium in drinking water: a public health threat. Arch Toxicol. https://doi.org/10.1007/s00204-020-02676-8
Chen A, Shang C, Shao J, Zhang J, Huang H (2017) The application of iron-based technologies in uranium remediation: a review. Sci Total Environ 575:1291–1306
Xie Y, Chen C, Ren X, Wang X, Wang H, Wang X (2019) Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Prog Mater Sci 103:180–234
Li H, Zhai F, Gui D, Wang X, Wu C, Zhang D, Dai X, Deng H, Su X, Diwu J, Lin Z, Chai Z, Wang S (2019) Powerful uranium extraction strategy with combined ligand complexation and photocatalytic reduction by postsynthetically modified photoactive metal-organic frameworks. Appl Catal B Environ 254:47–54
Abbasizadeh S, Keshtkar AR, Mousavian MA (2013) Preparation of a novel electrospun polyvinyl alcohol/titanium oxide nanofiber adsorbent modified with mercapto groups for uranium(VI) and thorium(IV) removal from aqueous solution. Chem Eng J 220:161–171
Keshtkar AR, Irani M, Moosavian MA (2013) Removal of uranium (VI) from aqueous solutions by adsorption using a novel electrospun PVA/TEOS/APTES hybrid nanofiber membrane: comparison with casting PVA/TEOS/APTES hybrid membrane. J Radioanal Nucl Chem 295(1):563–571
Wei X, Liu Q, Zhang H, Lu Z, Liu J, Chen R, Li R, Li Z, Liu P, Wang J (2017) Efficient removal of uranium(vi) from simulated seawater using amidoximated polyacrylonitrile/FeOOH composites. Dalton Trans 46(45):15746–15756
Karmakar R, Sen K (2019) Role of biomolecules in selective extraction of U(VI) using an aqueous biphasic system. J Radioanal Nucl Chem 322(1):57–66
Su M, Tsang DCW, Ren X, Shi Q, Tang J, Zhang H, Kong L, La H, Song G, Chen D (2019) Removal of U(VI) from nuclear mining effluent by porous hydroxyapatite: evaluation on characteristics, mechanisms and performance. Environ Pollut 254(Pt A):112891
Baker MR, Coutelot FM, Seaman JC (2019) Phosphate amendments for chemical immobilization of uranium in contaminated soil. Environ Int 129:565–572
Dummi Mahadevan G, Zhao F (2017) A concise review on microbial remediation cells (MRCs) in soil and groundwater radionuclides remediation. J Radioanal Nucl Chem 314(3):1477–1485
Lakaniemi A-M, Douglas GB, Kaksonen AH (2019) Engineering and kinetic aspects of bacterial uranium reduction for the remediation of uranium contaminated environments. J Hazard Mater 371:198–212
Li X, Ding C, Liao J, Du L, Sun Q, Yang J, Yang Y, Zhang D, Tang J, Liu N (2016) Bioaccumulation characterization of uranium by a novel Streptomyces sporoverrucosus dwc-3. J Environ Sci 41:162–171
Li C, Wang M, Luo X (2019) Uptake of uranium from aqueous solution by Nymphaea tetragona Georgi: the effect of the accompanying heavy metals. Appl Radiat Isot 150:157–163
Lichtfouse E (ed) (2014) Sustainable agriculture reviews, vol 13. Springer International Publishing, Cham
Li R, Dong F, Yang G, Zhang W, Zong M, Nie X, Zhou L, Babar A, Liu J, Ram B, Fan C, Zeng Y (2019) Characterization of arsenic and uranium pollution surrounding a uranium mine in Southwestern China and phytoremediation potential. Pol J Environ Stud 29(1):173–185
Liu M, Luo L, Dong F, Wei H, Nie X, Zhang W, Hu W, Ding C, Wang P (2019) Characteristics and mechanism of uranium photocatalytic removal enhanced by chelating hole scavenger citric acid in a TiO2 suspension system. J Radioanal Nucl Chem 319(1):147–158
He H, Zong M, Dong F, Yang P, Ke G, Liu M, Nie X, Ren W, Bian L (2017) Simultaneous removal and recovery of uranium from aqueous solution using TiO2 photoelectrochemical reduction method. J Radioanal Nucl Chem 313(1):59–67
Islas H, Flores MU, Reyes IA, Juárez JC, Reyes M, Teja AM, Palacios EG, Pandiyan T, Aguilar-Carrillo J (2020) Determination of the dissolution rate of hazardous jarosites in different conditions using the shrinking core kinetic model. J Hazard Mater 386:121664
Kendall MR, Madden AS, Elwood Madden ME, Hu Q (2013) Effects of arsenic incorporation on jarosite dissolution rates and reaction products. Geochim Cosmochim Acta 112:192–207
Papike JJ, Karner JM, Shearer CK (2006) Comparative planetary mineralogy: implications of martian and terrestrial jarosite. A crystal chemical perspective. Geochim Cosmochim Acta 70(5):1309–1321
Xu Z, Lu B, Wu J, Zhou L, Lan Y (2013) Reduction of Cr (VI) facilitated by biogenetic jarosite and analysis of its influencing factors with response surface methodology. Mater Sci Eng C Mater Biol Appl 33(7):3723–3729
Duff MC, Coughlin JU, Hunter DB (2002) Uranium co-precipitation with iron oxide minerals. Geochim Cosmochim Acta 66(20):3533–3547
Aguilar-Carrillo J, Herrera-García L, Reyes-Domínguez IA, Gutiérrez EJ (2020) Thallium(I) sequestration by jarosite and birnessite: Structural incorporation vs surface adsorption. Environ Pollut 257:113492
Xu T, Zhu R, Shang H, Xia Y, Liu X, Zhang L (2019) Photochemical behavior of ferrihydrite-oxalate system: Interfacial reaction mechanism and charge transfer process. Water Res 159:10–19
Chen J, Browne WR (2018) Photochemistry of iron complexes. Coordin Chem Rev 374:15–35
Dontsova TA, Yanushevskaya EI, Nahirniak SV, Makarchuk OV, Ivanets AI, Roshchina MY, Kutuzova AS, Kulikov LM (2018) Directional control of the structural adsorption properties of clays by magnetite modification. J Nanomater 2018:1–9
Ivanets AI, Prozorovich VG, Kouznetsova TF, Radkevich AV, Zarubo AM (2016) Mesoporous manganese oxides prepared by sol-gel method: Synthesis, characterization and sorption properties towards strontium ions. Environ Nanotechnol Monit Manage 6:261–269
Chen M, He M, Liu M, Dong F, Wei H, Nie X (2019) Synergistic effects of electron shuttle AQS and Alcaligenes faecalis on photocatalytic removal of U(VI). J Radioanal Nucl Chem 322(2):731–742
Xu Z, Bai S, Liang J, Zhou L, Lan Y (2013) Photocatalytic reduction of Cr (VI) by citric and oxalic acids over biogenetic jarosite. Mater Sci Eng C Mater Biol Appl 33(4):2192–2196
Wang H, Bigham JM, Jones FS, Tuovinen OH (2007) Synthesis and properties of ammoniojarosites prepared with iron-oxidizing acidophilic microorganisms at 22–65°C. Geochim Cosmochim Acta 71(1):155–164
Liao Y, Zhou L, Liang J, Xiong H (2009) Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Mat Sci Eng C 29(1):211–215
Guo Z, Li Y, Wu W (2009) Sorption of U(VI) on goethite: effects of pH, ionic strength, phosphate, carbonate and fulvic acid. Appl Radiat Isot 67(6):996–1000
Li W, Troyer LD, Lee SS, Wu J, Kim C, Lafferty BJ, Catalano JG, Fortner JD (2017) Engineering nanoscale iron oxides for uranyl sorption and separation: optimization of particle core size and bilayer surface coatings. ACS Appl Mater Interfaces 9(15):13163–13172
Regenspurg S, Schild D, Schäfer T, Huber F, Malmström ME (2009) Removal of uranium (VI) from the aqueous phase by iron (II) minerals in presence of bicarbonate. Appl Geochem 24(9):1617–1625
Flores M, Reyes I, Palacios E, Patiño F, Juárez J, Reyes M, Teja A, Islas H, Gutiérrez E (2019) Kinetic analysis of the thermal decomposition of a synthetic mercury jarosite. Minerals 9(4):200
Drouet C, Navrotsky A (2003) Synthesis, characterization, and thermochemistry of K-Na-H3O jarosites. Geochim Cosmochim Acta 67(11):2063–2076
Zhu J, Gan M, Zhang D, Hu Y, Chai L (2013) The nature of Schwertmannite and Jarosite mediated by two strains of Acidithiobacillus ferrooxidans with different ferrous oxidation ability. Mater Sci Eng C Mater Biol Appl 33(5):2679–2685
Bedi A, Singh BR, Deshmukh SK, Aggarwal N, Barrow CJ, Adholeya A (2018) Development of a novel myconanomining approach for the recovery of agriculturally important elements from jarosite waste. J Environ Sci (China) 67:356–367
Jönsson J, Persson P, Sjöberg S, Lövgren L (2005) Schwertmannite precipitated from acid mine drainage: phase transformation, sulphate release and surface properties. Appl Geochem 20(1):179–191
Wazne M, Korfiatis GP, Meng X (2003) Carbonate effects on hexavalent uranium adsorption by iron oxyhydroxide. Environ Sci Technol 37(16):3619–3624
Nie X, Dong F, Liu N, Liu M, Zhang D, Kang W, Sun S, Zhang W, Yang J (2015) Subcellular distribution of uranium in the roots of Spirodela punctata and surface interactions. Appl Surf Sci 347:122–130
Winstanley EH, Morris K, Abrahamsen-Mills LG, Blackham R, Shaw S (2019) U(VI) sorption during ferrihydrite formation: underpinning radioactive effluent treatment. J Hazard Mater 366:98–104
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem E 74:25–48
Lagergren S (1898) About the theory of so-called adsorption of soluble substances, Kungliga Svenska Vetenskapsakad. Handlingar 24:1–39
Khanday WA, Marrakchi F, Asif M, Hameed BH (2017) Mesoporous zeolite-activated carbon composite from oil palm ash as an effective adsorbent for methylene blue. J Taiwan Inst Chem E 70:32–41
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Ivanets AI, Srivastava V, Kitikova NV, Shashkova IL, Sillanpää M (2017) Kinetic and thermodynamic studies of the Co (II) and Ni (II) ions removal from aqueous solutions by Ca-Mg phosphates. Chemosphere 171:348–354
Shashkova IL, Ivanets AI, Kitikova NV, Sillanpää M (2017) Effect of phase composition on sorption behavior of Ca-Mg phosphates towards Sr (II) ions in aqueous solution. J Taiwan Inst Chem E 80:787–796
Rashidi F, Sarabi RS, Ghasemi Z, Seif A (2010) Kinetic, equilibrium and thermodynamic studies for the removal of lead (II) and copper (II) ions from aqueous solutions by nanocrystalline. Superlattices Microstruct 48(6):577–591
Ivanets AI, Srivastava V, Roshchina MY, Sillanpää M, Prozorovich VG, Pankov VV (2018) Magnesium ferrite nanoparticles as a magnetic sorbent for the removal of Mn2+, Co2+, Ni2+ and Cu2+ from aqueous solution. Ceram Int 44(8):9097–9104
Weller C, Tilgner A, Bräuer P, Herrmann H (2014) Modeling the impact of iron-carboxylate photochemistry on radical budget and carboxylate degradation in cloud droplets and particles. Environ Sci Technol 48(10):5652–5659
Jia H, Chen H, Nulaji G, Li X, Wang C (2015) Effect of low-molecular-weight organic acids on photo-degradation of phenanthrene catalyzed by Fe (III)-smectite under visible light. Chemosphere 138:266–271
Weller C, Horn S, Herrmann H (2013) Photolysis of Fe (III) carboxylato complexes: Fe (II) quantum yields and reaction mechanisms. J Photoch Photobio A 268:24–36
Ivanets A, Roshchina M, Srivastava V, Prozorovich V, Dontsova T, Nahirniak S, Pankov V, Hosseini-Bandegharaei A, Nguyen Tran H, Sillanpää M (2019) Effect of metal ions adsorption on the efficiency of methylene blue degradation onto MgFe2O4 as Fenton-like catalysts. Colloid Surface A 571:17–26
Jiang D, Li Y, Wu Y, Zhou P, Lan Y, Zhou L (2012) Photocatalytic reduction of Cr (VI) by small molecular weight organic acids over schwertmannite. Chemosphere 89(7):832–837
Bagus PS, Nelin CJ, Ilton ES (2013) Theoretical modeling of the uranium 4f XPS for U(VI) and U(IV) oxides. J Chem Phys 139(24):244704
Schindler M, Hawthorne FC, Freund MS, Burns PC (2009) XPS spectra of uranyl minerals and synthetic uranyl compounds. I: The U 4f spectrum. Geochim Cosmochim Acta 73(9):2471–2487
Dickinson M, Scott TB (2010) The application of zero-valent iron nanoparticles for the remediation of a uranium-contaminated waste effluent. J Hazard Mater 178(1–3):171–179
Kushwaha S, Sreedhar B, Padmaja P (2012) XPS, EXAFS, and FTIR as tools to probe the unexpected adsorption-coupled reduction of U(VI) to U(V) and U(IV) on Borassus flabellifer-based adsorbents. Langmuir 28(46):16038–16048
Jiang B, Gong Y, Gao J, Sun T, Liu Y, Oturan N, Oturan MA (2019) The reduction of Cr (VI) to Cr (III) mediated by environmentally relevant carboxylic acids: state-of-the-art and perspectives. J Hazard Mater 365:205–226
Weller C, Horn S, Herrmann H (2013) Effects of Fe (III)-concentration, speciation, excitation-wavelength and light intensity on the quantum yield of iron (III)-oxalato complex photolysis. J Photoch Photobiol A 255:41–49
Acknowledgements
This research was supported by the National Basic Research Program of China (973 Program: 2014CB846003), National Nature Science Foundation of China (Grant numbers: 51974261, 41802037)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, H., Dong, F., Chen, M. et al. Removal of uranium by biogenetic jarosite coupled with photoinduced reduction in the presence of oxalic acid: a low-cost remediation technology. J Radioanal Nucl Chem 324, 715–729 (2020). https://doi.org/10.1007/s10967-020-07125-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07125-5