Abstract
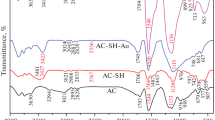
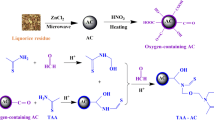
We present a simple strategy for preparing amidoxime modified activated carbon. The composition and morphology of the materials have been confirmed via powder XRD, BET, TGA, SEM and FT-IR studies. Batch adsorption experiment were exploited to explore adsorption performance of U(VI) by amidoxime-based activated carbon. The adsorption capacity of activated carbon was significantly improved after the modification by amidoxime, the amidoxime-based activated carbon was a possible adsorbent for adsorbing U(VI). This study reveals that with a facile synthesis and low-cost, amidoxime-based activated carbon can be regarded as a promising material for uranium-containing wastewater treatment.










Similar content being viewed by others
References
Wu F, Pu N, Ye G, Sun T, Wang Z, Song Y, Wang W, Huo X, Lu Y, Chen J (2017) Performance and mechanism of uranium adsorption from seawater to poly(dopamine)-inspired sorbents. Environ Sci Technol 51(8):4606–4614
Xiao J, Jing Y, Yao Y, Wang X, Jia Y (2019) Synthesis of amidoxime-decorated 3d cubic mesoporous silica via self-assembly co-condensation as a superior uranium(VI) adsorbent. J Mol Liq 277:843–855
Wang D, Xu Y, Xiao D, Qiao Q, Yin P, Yang Z, Li J, Winchester W, Wang Z, Hayat T (2019) Ultra-thin iron phosphate nanosheets for high efficient U(VI) adsorption. J Hazard Mater 371:83–93
Wang Y, Huang H, Duan S, Liu X, Sun J, Hayat T, Alsaedi A, Li J (2017) A new application of a mesoporous hybrid of tungsten oxide and carbon as an adsorbent for elimination of \({\text{ Sr}^{2+}}\) and \({\text{ Co}^{2+}}\) from an aquatic environment. ACS Sustain Chem Eng 6(2):2462–2473
Zhao Y, Wang X, Li J, Wang X (2015) Amidoxime functionalization of mesoporous silica and its high removal of U(VI). Polym Chem 6(30):5376–5384
Yuan D, Chen L, Xiong X, Yuan L, Liao S, Wang Y (2016) Removal of uranium(VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheres prepared by a controlled radical polymerization in the presence of DPE. Chem Eng J 285:358–367
Zhang M, Gao Q, Yang C, Pang L, Wang H, Li H, Li R, Xu L, Xing Z, Hu J et al (2016) Preparation of amidoxime-based nylon-66 fibers for removing uranium from low-concentration aqueous solutions and simulated nuclear industry effluents. Ind Eng Chem Res 55(40):10523–10532
Zhao C, Liu J, Yuan G, Liu J, Zhang H, Yang J, Yang Y, Liu N, Sun Q, Liao J (2018) A novel activated sludge-graphene oxide composites for the removal of uranium(VI) from aqueous solutions. J Mol Liq 271:786–794
Fan Qh, Li P, Yf Chen, Ws Wu (2011) Preparation and application of attapulgite/iron oxide magnetic composites for the removal of U(VI) from aqueous solution. J Hazard Mater 192(3):1851–1859
Zhao G, Huang X, Tang Z, Huang Q, Niu F, Wang X (2018) Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem 9(26):3562–3582
Yin L, Song S, Wang X, Niu F, Ma R, Yu S, Wen T, Chen Y, Hayat T, Alsaedi A et al (2018) Rationally designed core-shell and yolk-shell magnetic titanate nanosheets for efficient U(VI) adsorption performance. Environ Pollut 238:725–738
Bq Lu, Li M, Xw Zhang, Huang Cm Wu, Xy Fang Q (2018) Immobilization of uranium into magnetite from aqueous solution by electrodepositing approach. J Hazard Mater 343:255–265
Dutta DP, Nath S (2018) Low cost synthesis of \({\text{SiO}_{2} /\text{ C }}\) nanocomposite from corn cobs and its adsorption of uranium(VI), chromium(VI) and cationic dyes from wastewater. J Mol Liq 269:140–151
Ma D, Hu S, Li Y, Xu Z (2019) Adsorption of uranium on phosphoricacid-activated peanut shells. Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1606016
Zhao Y, Liu C, Feng M, Chen Z, Li S, Tian G, Wang L, Huang J, Li S (2010) Solid phase extraction of uranium(VI) onto benzoylthiourea-anchored activated carbon. J Hazard Mater 176(1–3):119–124
Starvin A, Rao TP (2004) Solid phase extractive preconcentration of uranium(VI) onto diarylazobisphenol modified activated carbon. Talanta 63(2):225–232
Saleh TA, Tuzen M, Sarı A et al (2017) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227
Xie L, Wang Y, Wang Y, Li X, Tian Q, Liu D, Sun G, Wang X (2018) Study of poly(acrylamidoxime) brushes conformation with uranium adsorption by neutron reflectivity. Mater Lett 220:47–49
Ladshaw AP, Wiechert AI, Das S, Yiacoumi S, Tsouris C (2017) Amidoxime polymers for uranium adsorption: influence of comonomers and temperature. Materials 10(11):1268
Bai J, Yao H, Fan F, Lin M, Zhang L, Ding H, Lei F, Wu X, Li X, Guo J et al (2010) Biosorption of uranium by chemically modified rhodotorula glutinis. J Environ Radioact 101(11):969–973
Wang Y, Wang Z, Ang R, Yang J, Liu N, Liao J, Yang Y, Tang J (2015) Synthesis of amidoximated graphene oxide nanoribbons from unzipping of multiwalled carbon nanotubes for selective separation of uranium(VI). RSC Adv 5(108):89309–89318
Wang X, Ji G, Zhu G, Song C, Zhang H, Gao C (2019) Surface hydroxylation of SBA-15 via alkaline for efficient amidoxime-functionalization and enhanced uranium adsorption. Sep Purif Technol 209:623–635
Zhang Z, Dong Z, Wang X, Ying D, Niu F, Cao X, Wang Y, Hua R, Liu Y, Wang X (2018) Ordered mesoporous polymer-carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chem Eng J 341:208–217
Aljarrah M, Al-Harahsheh MS, Mayyas M, Alrebaki M (2018) In situ synthesis of quaternary ammonium on silica-coated magnetic nanoparticles and it’s application for the removal of uranium(VI) from aqueous media. J Environ Chem Eng 6(5):5662–5669
Li W, Liu Q, Liu J, Zhang H, Li R, Li Z, Jing X, Wang J (2017) Removal U(VI) from artificial seawater using facilely and covalently grafted polyacrylonitrile fibers with lysine. Appl Surf Sci 403:378–388
Ma F, Dong B, Gui Y, Cao M, Han L, Jiao C, Lv H, Hou J, Xue Y (2018) Adsorption of low-concentration uranyl ion by amidoxime polyacrylonitrile fibers. Ind Eng Chem Res 57(51):17384–17393
Xie CY, Jing SP, Wang Y, Lin X, Bao HL, Guan CZ, Jin C, Wang JQ (2017) Adsorption of uranium(VI) onto amidoxime-functionalized ultra-high molecular weight polyethylene fibers from aqueous solution. Nucl Sci Technol 28(7):94
Heshmati H, Torab-Mostaedi M, Ghanadzadeh Gilani H, Heydari A (2015) Kinetic, isotherm, and thermodynamic investigations of uranium(VI) adsorption on synthesized ion-exchange chelating resin and prediction with an artificial neural network. Desalination Water Treat 55(4):1076–1087
Tran HN, You SJ, Hosseini-Bandegharaei A, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Wei X, Liu Q, Zhang H, Liu J, Chen R, Li R, Li Z, Liu P, Wang J (2018) Rapid and efficient uranium(VI) capture by phytic acid/polyaniline/\(\text{ FeOOH }\) composites. J Colloid Interface Sci 511:1–11
Guibal E, Milot C, Tobin JM (1998) Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Ind Eng Chem Res 37(4):1454–1463
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57(1):385–470
Yang P, Liu Q, Liu J, Chen R, Li R, Bai X, Wang J (2019) Highly efficient immobilization of uranium(VI) from aqueous solution by phosphonate-functionalized dendritic fibrous nanosilica (DFNS). J Hazard Mater 363:248–257
Dabrowski A (2001) Adsorption-from theory to practice. Adv Colloid Interface Sci 93(1–3):135–224
Yin Z, Xiong J, Chen M, Hu S, Cheng H (2016) Recovery of uranium(VI) from aqueous solution by amidoxime functionalized wool fibers. J Radioanal Nucl Chem 307(2):1471–1479
Hazer O, Kartal Ş (2010) Use of amidoximated hydrogel for removal and recovery of U(VI) ion from water samples. Talanta 82(5):1974–1979
Duan S, Xu X, Liu X, Wang Y, Hayat T, Alsaedi A, Meng Y, Li J (2018) Highly enhanced adsorption performance of U(VI) by non-thermal plasma modified magnetic \({\text{Fe}_{3}\text{O}_{4}}\) nanoparticles. J Colloid Interface Sci 513:92–103
Imam EA, El-Sayed IET, Mahfouz MG, Tolba AA, Akashi T, Galhoum AA, Guibal E (2018) Synthesis of \(\alpha\)-aminophosphonate functionalized chitosan sorbents: effect of methyl vs phenyl group on uranium sorption. Chem Eng J 352:1022–1034
Zhang Z, Duan S, Chen H, Zhang F, Hayat T, Alsaedi A, Li J (2018) Synthesis of porous magnetic \({\text{Ni}_{0.6}\text{Fe}_{2.4}\text{O}_{4}}\) nanorods for highly efficient adsorption of U(VI). J Chem Eng Data 63(5):1810–1820
Li P, Wang J, Wang X, He B, Pan D, Liang J, Wang F, Fan Q (2018) Arsenazo-functionalized magnetic carbon composite for uranium(VI) removal from aqueous solution. J Mol Liq 269:441–449
Yang P, Zhang H, Liu Q, Liu J, Chen R, Yu J, Hou J, Bai X, Wang J (2019) Nano-sized architectural design of multi-activity graphene oxide (GO) by chemical post-decoration for efficient uranium(VI) extraction. J Hazard Mater 375:320–329
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47(17):9904–9910
Nakkeeran E, Selvaraju N (2017) Biosorption of chromium(VI) in aqueous solutions by chemically modified strychnine tree fruit shell. Int J Phytorem 19(12):1065–1076
Acknowledgements
The authors gratefully acknowledge support from the Foundation of Heilongjiang Postdoctoral Science Foundation (LBH-Z17050), the China Postdoctoral Science Foundation (2019M651257), the National Natural Science Foundation of China (21771045), the Fundamental Research Funds for the Central Universities (3072019CFJ1501), and the Decommissioning of Nuclear Facilities and special funds for radioactive waste management ([2017]955). And the authors acknowledged the Innovation Center of Nuclear Materials for National Defense Industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, P., Yu, Q., Xue, Y. et al. Adsorption performance of U(VI) by amidoxime-based activated carbon. J Radioanal Nucl Chem 324, 813–822 (2020). https://doi.org/10.1007/s10967-020-07111-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07111-x