Abstract
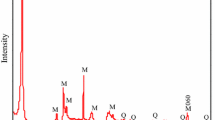
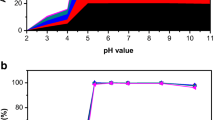
Montmorillonite colloid was synthesized and characterized and the adsorption of U(VI) on colloid as a function of contact time, temperature, initial concentration of U(VI) solution, pH and foreign ions was investigated through batch experiments. The results illustrate that the adsorption reaches equilibrium quickly within 30 mins. Results showed that the absorption process is influenced by pH value, temperature and ions, among which pH value is the most significant. Adsorption percentage increases obviously at pH = 4.0–7.0, and then decreases with the increase of pH value. The competitive cations also inhibit the adsorption. pH value and ions affect the surface properties and charges of the colloid and the chemical form of nuclides. The experimental data was analyzed in detail. The maximum adsorption capacity obtained by fitting the second-order kinetic model is very close to the experimental data. The Langmuir model fits the experimental data better than Freundlich models, R2 = 0.991. The results of fitting two adsorption isotherms show that the adsorption of colloids on uranium has both physical and chemical adsorption, and is mainly monolayer adsorption. Furthermore, the adsorption process is a spontaneous endothermic reaction by calculating the thermodynamic parameters. This experiment can provide some data basis for the adsorption study of U(VI) on colloids.







Similar content being viewed by others
References
Wang Ju (2014) On area-specific underground research laboratory for geological disposal of high-level radioactive waste in China. J Rock Mech Geotech Eng 6(02):99–104
Wang Ju, Chen Liang, Rui Su, Zhao Xingguang (2018) The Beishan underground research laboratory for geological disposal of high-level radioactive waste in China: planning, site selection, site characterization and in situ tests. J Rock Mech Geotech Eng 10(03):411–435
Novikov AP, Kalmykov SN, Utsunomiya S (2006) Colloid transport of plutonium in the far-field of the Mayak Production Association, Russia. Science 314:638–641
Kersting AB, Efurd DW, Finnegan DL (1999) Migration of plutonium in ground water at the Nevada Test Site. Nature 397:56–59
Albarran N, Missana T, García-Gutiérrez Miguel (2011) Strontium migration in a crystalline medium: effects of the presence of bentonite colloids. J Contam Hydrol 122:76–85
Champ DR, Merritt WF, Young JL (1981) Potential for the rapid transport of plutonium in groundwater as demonstrated by core column studies. MRS Proc 11:745
Matshunaga T, Nagao S, Ueno T, Takeda S, Amano H, Tkachenko Y (2004) Association of dissolved radionuclides released by the Chernoyl accident with colloidal materials in surface water. Appl Geochem 19:1581–1599
Kunze P, Seher H, Hauser W (2008) The influence of colloid formation in a granite groundwater bentonite porewater mixing zone on radionuclide speciation. J Contam Hydrol 102:263–272
Möri A, Alexander WR, Geckeis H (2003) The colloid and radionuclide retardation experiment at the Grimsel test site: influence of bentonite colloids on radionuclide migration in a fractured rock. J Colloids Surf A 217:33–47
Buddemeier RW, Hunt JR (1988) Transport of colloidal contaminants in groundwater: radionuclide migration at the Nevada test site. J Appl Geochem 3:535–548
Gavrilescu M, Pavel LV, Cretescu I (2009) Characterization and remediation of soils contaminated with uranium. J Hazard Mater 163(2–3):475–510
Kang MJ, Han BE, Hahn PS (2002) Precipitation and adsorption of uranium(VI) under various aqueous conditions. Environ Eng Res 7(3):5743–5753
Belgacem A, Rebiai R, Hadoun H (2014) The removal of uranium(VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ Sci Pollut Res 21(1):684–694
Joseph C, Schmeide K, Sachs S (2011) Sorption of uranium(VI) onto opalinus clay in the absence and presence of humic acid in opalinus clay pore water. Chem Geol 284(3–4):0–250
Korichi S, Bensmaili A (2009) Sorption of uranium(VI) on homoionic sodium smectite experimental study and surface complexation modeling. J Hazard Mater 169(1–3):780–793
Claveranne-Lamolère Céline, Lespes G, Dubascoux Stéphane (2009) Colloidal transport of uranium in soil: size fractionation and characterization by field-flow fractionation-multi-detection. J Chromatogr A 1216(52):9113–9119
Lesher EK, Ranville JF, Honeyman BD (2009) Analysis of pH dependent uranium(vi) sorption to nanoparticulate hematite by flow field-flow fractionation-inductively coupled plasma mass spectrometry. Environ Sci Technol 43(14):5403–5409
Guo L, Warnken KW, Santschi PH (2007) Retention behavior of dissolved uranium during ultrafiltration: implications for colloidal U in surface waters. Mar Chem 107(2):156–166
Sun H, Yin X, Cao X (2012) Effect on the migration and release of montmorillonite colloid in saturated porous media. J Environ Sci 1120–1125 (in China)
Singh BP, Menchavez R, Takai C, Fuji M, Takahashi M (2005) Stability of dispersions of colloidal alumina particles in aqueous suspensions. J Colloid Interface Sci 291(1):181–186
Sun J, Velamakanni BV, Gerberich WW, Francis LF (2004) Aqueous latex/ceramic nanoparticle dispersions: colloidal stability and coating properties. J Colloid Interface Sci 280:387–399
Uriev NB (2017) Dynamic aggregative and structural stability of high-concentration colloid dispersed systems. Prot Met Phys Chem Surf 53(1):188–197
Tuddenham WM, Lyon RJP (1960) Infrared techniques in the identification and measurement of minerals. Anal Chem 32(12):1630–1634
Tan XL, Hu J, Montavon G (2011) Adsorption speciation of nickel(II) onto ca-montmorillonite: batch, EXAFS techniques and modeling. J Dalton Trans 40:10953
Anirudhan TS, Radhakrishnan PG (2009) Kinetics, thermodynamics and surface heterogeneity assessment of uranium(VI) adsorption onto cation exchange resin derived from a lignocellulosic residue. Appl Surf Sci 255(9):4983–4991
Tunali S, Akar T, Zcan AS (2006) Equilibrium and kinetics of biosorption of lead (II) from aqueous solutions by Cephalosporium aphidicola. Sep Purif Technol 47(3):105–112
Missana T, Alonso Ú, Turrero MJ (2003) Generation and stability of bentonite colloids at the bentonite/granite interface of a deep geological radioactive waste repository. J Contam Hydrol 61(1):17–31
Tan L, Jin Y, Chen J (2011) Sorption of radiocobalt(II) from aqueous solutions to Na-attapulgite. J Radioanal Nucl Chem 289(2):601–610
Zhang C, Liu Z, Chen L (2012) Influence of pH, humic acid, ionic strength, foreign ions, and temperature on 60Co(II) adsorption onto γ-Al2O3. J Radioanal Nucl Chem 292:411–419
Song X, Wang Y, Cai J (2013) Interaction of U(VI) with Na-attapulgite in the presence and absence of humic acid as a function of pH, ionic strength and temperature. J Radioanal Nucl Chem 295:685–695
Fan Q, Shao D, Lu Y (2009) Effect of pH, ionic strength, temperature and humic substances on the adsorption of Ni (II) to Na-attapulgite. Chem Eng J 150:188–195
Fan F-L, Qin Z, Bai J, Rong W-D, Fan F-Y, Tian W, Wu X-L, Wang Y, Zhao L (2012) Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. J Environ Radioact 106:40–46
Zcan A, Minencü E, Zcan AS (2006) Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf A 77(1–3):90–97
Karthikeyan T, Rajgopal S, Miranda LR (2005) Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J Hazard Mater 124(1–3):192–199
Aytas S, Yurtlu M, Donat R (2009) Adsorption characteristic of U(VI) ion onto thermally activated bentonite. J Hazard Mater 172:667–674
Kraepiel AML, Keller K, Morel FMM (1999) A model for metal adadsorption on montmorillonite. J Colloid Interface Sci 210:43–54
Batchelor Bill (1998) Leach models for contaminants immobilized by pH-dependent mechanisms. J Environ Sci Technol 32:1721–1726
Zachara JM, Mckinley JP (1993) Influence of hydrolysis on the adsorption of metal cations by smectites: importance of edge coordination reactions—dedicated to Paul W. Schindler on his retirement. J Aquat Sci 55:250–261
Lagaly G, Ziesmer S (2003) Colloid chemistry of clay minerals: the coagulation of montmorillonite dispersions. J Adv Colloid Interface Sci 100:105–128
Yoshida T, Suzuki M (2006) Migration of strontium and europium in quartz sand column in the presence of humic acid: effect of ionic strength. J Radioanal Nucl Chem 270:363–368
Prikryl JD, Jain A, Turner DR (2001) Uranium(VI) adsorption behavior on silicate mineral mixture. J Contam Hydrol 47:241–253
Acknowledgements
Financial supports from National Natural Science Foundation of China Youth Fun (No. 41603124) and National Natural Science Foundation of China (No. 41630646) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, S., Ma, J., Shi, Y. et al. Uranium(VI) adsorption on montmorillonite colloid. J Radioanal Nucl Chem 324, 541–549 (2020). https://doi.org/10.1007/s10967-020-07083-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07083-y