Abstract
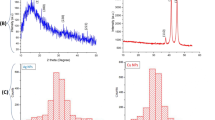
Uranium is carcinogenic ion and if present in water above its permissible limit, is very harmful to living beings. The main objective of this study is the removal of uranium from water sample using nano zero-valent copper prepared by green synthesis from Anacardium occidentale testa extract by a fully environment-friendly and cost-effective process without involvement of any hazardous chemicals. The formation of the A. occidentale-Cu nanoparticle is characterized through UV–Visible spectroscopy, FTIR, SEM–EDS, XRD and HR-TEM analysis. The adsorption capacity of copper nanoparticles, effect of pH, adsorbent and adsorbate dosage and contact time are studied and analyzed by Langmuir and Freundlich adsorption isotherm.







Similar content being viewed by others
References
Zhu KR, Chen CL, Xu MWC, Chen K, Tan XL, Wakeel M, Alharbi NS (2018) In situ carbothermal reduction synthesis of Fe nanocrystals embedded into N-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction. Chem Eng J 331:395–405
Sun YB, Wang XX, Ai YJ, Yu ZM, Huang W, Chen CL, Hayat T, Alsaedi A, Wang XK (2017) Interaction of sulfonated graphene oxide with U(VI) studied by spectroscopic analysis and theoretical calculations. Chem Eng J 310:292–299
Chen LL, Feng SJ, Zhao DL, Chen SH, Li FF, Chen CL (2017) Efficient sorption and reduction of U(VI) on zero-valent iron-polyaniline-graphene aerogel ternary composite. J Colloid Interface Sci 490:197–206
Kalita MPC, Deka K, Das J, Hazarika N, Dey P, Das R, Paul S, Sarmah T, Sarma BK (2012) X-ray diffraction line profile analysis of chemically synthesizedlead sulphide nanocrystals. Mater Lett 87:84–86
Ajitha B, Reddy YAK, Kim MJ, Jeon H-J, Ahn CW (2016) Superior catalytic activity of synthesized triangular silver nanoplates with optimized sizes andshapes. Catal Sci Technol 6:8289–8299
Jain PK, Huang X, El-Sayed IH, El-Sayed MA (2008) Noble metals on the nanoscale: optical and photo thermal properties and some applications inimaging, sensing, biology and medicine. Acc Chem Res 41:1578–1586
Nasrollahzadeh M, Zahraei A, Ehsani A, Khalaj M (2014) Synthesis characterization, antibacterial and catalytic activity of a nanopolymer supportedcopper(II) complex as a highly active and recyclable catalyst for the formamidation of arylboronic acids under aerobic conditions. RSC Adv 4:20351–20357
Yi Y, Tu G, Tsang PE, Xiao S, Fang Z (2019) Green synthesis of iron-based nanoparticles from extracts of Nephrolepis auriculata and applications for Cr(VI) removal. Mater Lett 234:388–391
Ali JA, Kolo K, Sajadi SM, Manshad AK, Stephen KD (2019) Green synthesis of ZnO/SiO2 nanocomposite from pomegranate seed extract: coating by natural xanthan polymer and its characterisations. Micro Nano Lett 14:638–641
Ismail M, Gul S, Khan MI, Khan MA, Asiri AM, Khan SB (2019) Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process Synth 8(1):135–143
Roy K, Ghosh CK, Sarkar CK (2019) Rapid detection of hazardous H2O2 by biogenic copper nanoparticles synthesized using Eichhornia crassipes extract. Microsyst Technol 25(5):1699–1703
Ahmed A, Usman M, Liu QY, Shen YQ, Yu B, Cong HL (2019) Plant mediated synthesis of copper nanoparticles by using Camelia sinensis leaves extract and their applications in dye degradation. Ferroelectrics 549(1):61–69
Sebeia N, Jabli M, Ghith A, Saleh TA (2019) Eco-friendly synthesis of Cynomorium coccineum extract for controlled production of copper nanoparticles for sorption of methylene blue dye. Arab J Chem 13:4263–4274
Sebeia N, Jabli M, Ghith A (2019) Biological synthesis of copper nanoparticles, using Nerium oleander leaves extract: characterization and study of their interaction with organic dyes. Inorg Chem Commun 105:36–46
Ismail NA, Shameli K, Wong MMT, Teow SY, Chew J, Sukri SNAM (2019) Antibacterial and cytotoxic effect of honey mediated copper nanoparticles synthesized using ultrasonic assistance. Mater Sci Eng, C 104:109899
Chandrasekara N, Shahidi F (2011) Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J Agric Food Chem 59:5006–5014
Chandrasekara N, Shahidi F (2011) Antioxidative potential of cashew phenolics in food and biological model systems as affected by roasting. Food Chem 129:1388–1396
Mathew AG, Parpia HAB (1970) Polyphenols of cashew kernel testa. J Food Sci 35:140–143
Trox J, Vadivel V, Vetter W, Stuetz W, Kammerer DR, Carle R, Scherbaum V, Gola U, Nohr D, Biesalski HK (2011) Food Chem 128:1094–1099
Edison TNJI, Atchudan R, Sethuraman MG, Lee YR (2016) Reductive-degradation of carcinogenic azo dyes using Anacardium occidentale testa derived silver nanoparticles. J Photochem Photobiol, B 162:604–610
Chandra C, Khan F, Agrawal S, Soni VK (2020) Removal of Uranium(VI) from aqueous solution by iron nanoparticles synthesized from testa extract of Annacardium occidentale: a fluorimetric study. Asian J Chem. https://doi.org/10.14233/ajchem.2020.22287
Nasrollahzadeh M, Sajadi SM, Khalaj M (2014) Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L. and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv 4(88):47313–47318
Nasrollahzadeh M, Momeni SS, Sajadi SM (2017) Green synthesis of copper nanoparticles using Plantago asiatica leaf extract and their application for the cyanation of aldehydes using K4Fe (CN) 6. J Colloid Interface Sci 506:471–477
Christian P, Bromfield M (2010) Preparation of small silver, gold and copper nanoparticles which disperse in both polar and non-polar solvents. J Mater Chem 20(6):1135–1139
Theivasanthi T, Alagar M (2010) X-ray diffraction studies of copper nanopowder. arXiv preprint arXiv:1003.6068
Sharma P, Pant S, Dave V, Tak K, Sadhu V, Reddy KR (2019) Green synthesis and characterization of copper nanoparticles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J Microbiol Methods 160:107–116
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies. Bioresour Technol 96(11):1241–1248
Xiao-teng Z, Dong-mei J, Yi-qun X, Jun-chang C, Shuai H, Liang-shu X (2019) Adsorption of Uranium (VI) from aqueous solution by modified rice stem. J Chem 2019:1–10
Sylwester ER, Hudson EA, Allen PG (2000) The structure of uranium (VI) sorption complexes on silica, alumina, and montmorillonite. Geochim Cosmochim Acta 64:2431–2438
Akyil S, Eral M (2005) Preparation of composite adsorbents and their characteristics. J Radioanal Nucl Chem 266:89–93
Ladeira AC, Goncalves CR (2007) Influence of anionic species on uranium separation from acid mine water using strong base resins. J Hazard Mater 148:499–504
Xie SB, Zhang C, Zhou XH, Yang J, Zhang XJ, Song WJ (2009) Removal of uranium (VI) from aqueous solution by adsorption of hematite. J Environ Radioact 100:162–166
Wang GH, Liu JS, Wang XG, Xie ZY, Deng NS (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Kutahyalı C, Eral M (2010) Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. J Nucl Mater 396:251–256
Fan FL, Qin Z, Bai J, Rong WD, Fan FY, Tian W, Wu XL, Wang Y, Zhao L (2012) Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. J Environ Radioact 106:40–46
Zong PF, Wang SF, Zhao YL, Wang H, Pan H, He CH (2013) Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem Eng J 220:45–52
Abd El-Magied MO (2016) Sorption of uranium ions from their aqueous solution by resins containing nanomagnetite particles. J Eng 2016:1–11
Abdi S, Nasiri M, Mesbahi A, Khani MH (2017) Investigation of uranium (VI) adsorption by polypyrrole. J Hazard Mater 332:132–139
Wu Y, Chen D, Kong L, Tsang DC, Su M (2019) Rapid and effective removal of uranium (VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. J Hazard Mater 371:397–405
Chaudhary M, Singh L, Rekha P, Srivastava VC, Mohanty P (2019) Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine. Chem Eng J 378:122236
Acknowledgements
The authors are thankful to Dr. A. M. Rawani, Director, National Institute of Technology, Raipur for facilities of laboratory and library. One of the authors Ch Chandra gratefully acknowledges the JRF awarded by UGC (Grant No. 3673/(NET-DEC.2013)), New Delhi, and SAIF, IIT Bombay for providing the facility for HR-TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chandra, C., Khan, F. Nano-scale zerovalent copper: green synthesis, characterization and efficient removal of uranium. J Radioanal Nucl Chem 324, 589–597 (2020). https://doi.org/10.1007/s10967-020-07080-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07080-1