Abstract
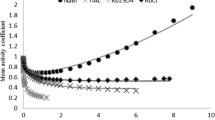
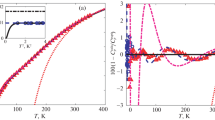
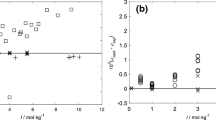
The activity and osmotic coefficients of fission product systems CsOH + CsCl, CsOH + CsBr and CsOH + CsI are of importance for studying the removal of cesium ions from radioactive wastewater and the hygroscopic growth of its aerosols. Various mixtures of alkali metal hydroxides and their halides are selected as the verification systems. Results indicate that the prediction effects of electrolyte MIVM (eMIVM) are better than that of Pitzer’s equation. Then, the activity coefficient, osmotic coefficient and excess Gibbs energy of the above mentioned systems are further predicted by both models. It is found that the predicted values of eMIVM are more reliable and reasonable.












Similar content being viewed by others
References
Wang JL, Zhuang ST (2019) Removal of cesium ions from aqueous solutions using various separation technologies. Rev Environ Sci Bio-Technol 18:231–269
Park Y, Lee YC, Shin WS, Choi SJ (2010) Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate–polyacrylonitrile (AMP–PAN). Chem Eng J 162:685–695
Nilchi A, Saberi R, Moradi M, Azizpour H, Zarghami R (2011) Adsorption of cesium on copper hexacyanoferrate–PAN composite ion exchanger from aqueous solution. Chem Eng J 172:572–580
Alby D, Charnay C, Heran M, Prelot B, Zajac J (2018) Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: synthesis and shaping, sorption capacity, mechanisms, and selectivity—a review. J Hazard Mater 344:511–530
Zhang XY, Gu P, Liu Y (2019) Decontamination of radioactive wastewater: state of the art and challenges forward. Chemosphere 215:543–553
Mishra G, Mandariya AK, Tripathi SN, Mariam Joshi M, Khan A, Sapra BK (2019) Hygroscopic growth of CsI and CsOH particles in context of nuclear reactor accident research. J Aerosol Sci 132:60–69
Harned HS, Schupp OE Jr (1930) The activity coefficient and dissociation of water in cesium chloride solutions. J Am Chem Soc 52:3892–3900
Pitzer KS (1973) Thermodynamics of electrolytes. I. Theoretical basis and general equations. J Phys Chem 77:268–277
Pitzer KS, Kim JJ (1974) Thermodynamics of electrolytes. IV. Activity and osmotic coefficients for mixed electrolytes. J Am Chem Soc 96:5701–5707
Kunz W (2009) Specific ion effects. World Scientific Publishing Company, Singapore
Tao DP (2000) A new model of thermodynamics of liquid mixtures and its application to liquid alloys. Thermochim Acta 363:105–113
Chen CC, Britt HI, Boston JF, Evans LB (1982) Local composition model for excess Gibbs energy of electrolyte systems. Part I: single solvent, single completely dissociated electrolyte systems. AIChE J 28:588–596
Chen CC, Evans LB (1986) A local composition model for the excess Gibbs energy of aqueous electrolyte systems. AIChE J 32:444–454
Pitzer KS (1980) Electrolytes. From dilute solutions to fused salts. J Am Chem Soc 102:2902–2906
Haghtalab A, Peyvandi K (2009) Electrolyte-UNIQUAC-NRF model for the correlation of the mean activity coefficient of electrolyte solutions. Fluid Phase Equilib 281:163–171
Robinson RA, Stokes RH (2002) Electrolyte solutions, 2nd edn. Dover Publications, New York
Rard JA, Miller DG (1982) Isopiestic determination of the osmotic and activity coefficients of aqueous cesium chloride, strontium chloride, and mixtures of sodium chloride and cesium chloride at 25°C. J Chem Eng Data 27:169–173
Pitzer KS, Mayorga G (1973) Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J Phys Chem 77:2300–2308
Falciola L, Mussini PR, Mussini T, Pozzi S, Rondinini S (2003) A thermodynamic study of the aqueous (sodium chloride + sodium hydroxide) electrolyte using sodium amalgam and thallous chloride electrode cells. J Chem Thermodyn 35:405–416
Königsberger E, Königsberger LC, Hefter G, May PM (2007) Zdanovskii’s rule and isopiestic measurements applied to synthetic Bayer liquors. J Solut Chem 36:1619–1634
Harned HS, Swindells FE (1926) The activity coefficient of lithium hydroxide in water and in aqueous lithium chloride solutions, and the dissociation of water in lithium chloride solutions. J Am Chem Soc 48:126–135
Harned HS, Cook MA (1937) The activity and osmotic coefficients of some hydroxide—chloride mixtures in aqueous solution. J Am Chem Soc 59:1890–1893
Harned HS (1925) The activity coefficient of sodium hydroxide in sodium chloride solutions. J Am Chem Soc 47:684–689
Harned HS, Harris JM Jr (1928) The activity coefficients of sodium and potassium hydroxides in their corresponding chloride solutions at high constant total molality. J Am Chem Soc 50(10):2633–2637
Harned HS, James GM (1926) The dissociation of water in potassium and sodium bromide solutions. J Phys Chem 30:1060–1072
Rowland D, May PM (2016) An investigation of Harned’s rule for predicting the activity coefficients of strong aqueous electrolyte solution mixtures at 25°C. J Chem Eng Data 62:310–327
Kim HT, Frederick WJ Jr (1988) Evaluation of Pitzer ion interaction parameters of aqueous mixed electrolyte solutions at 25°C. 2. Ternary mixing parameters. J Chem Eng Data 33:278–283
Lach A, André L, Lassin A, Azaroual M, Serin JP, Cezac P (2015) A new Pitzer parameterization for the binary NaOH–H2O and ternary NaOH–NaCl–H2O and NaOH–LiOH–H2O systems up to NaOH solid salt saturation, from 273.15 to 523.15 K and at saturated vapor pressure. J Solut Chem 44:1424–1451
Huang XT, Li SN, Zhai Q, Jiang YC, Hu MC (2016) Thermodynamic studies of (RbF + RbCl + H2O) and (CsF + CsCl + H2O) ternary systems from potentiometric measurements at T = 298.2 K. J Chem Thermodyn 103:157–164
Dou ZD, Li SN, Zhai QG, Jiang YC, Hu MC (2018) Potentiometric investigation of the thermodynamic properties of mixed electrolyte systems at 298.2 K: CsF + CsBr + H2O and CsF + CsNO3 + H2O. J Chem Eng Data 63:3801–3808
Marcus Y (1991) Thermodynamics of solvation of ions. Part 5. —Gibbs free energy of hydration at 298.15 K. J Chem Soc Faraday Trans 87:2995–2999
Lee LL (2008) Molecular thermodynamics of electrolyte solutions. World Scientific, Singapore
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China under Grant No. 51464022.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C., Xing, Y. & Tao, D. Prediction of activity and osmotic coefficients of fission product systems CsOH + CsX (X = Cl, Br, I) at 298.15 K. J Radioanal Nucl Chem 323, 773–784 (2020). https://doi.org/10.1007/s10967-019-06989-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06989-6