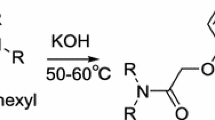
Abstract
The extraction behavior and separation performance of Re(VII) and U(VI) from the nitric acid medium were studied respectively, in which an asymmetrical diglycolamides, N,N′-dimethyl-N,N′-dioctyldiglycolamide (DMDODGA) was used as the extractant, and 40/60(v/v)% n-octanol/kerosene was used as the diluent. The effects of the concentration of the aqueous HNO3, the initial metal ion and the extractant on the distribution ratios (DRe and DU) and the separation factor (βU/Re) were discussed. Formation of the 1:1 and 1:2 Re/DODMDGA and 1:1 U/DMDODGA complexes were also analyzed using the slope analysis method. Besides, βU/Re increases as the aqueous HNO3 concentration increases.












Similar content being viewed by others
References
Sood DD, Patil SK (1996) Chemistry of nuclear fuel reprocessing: current status. J Radioanal Nucl Chem 203:547–573
Mathur JN, Murali MS, Nash KL (2001) Actinide partitioning-a review. Solv Extr Ion Exch 19:357–390
Eccles H (2000) Nuclear fuel cycle technologies -sustainable in the twenty first century? Solv Extr Ion Exch 18:633–654
Sasaki Y, Sugo Y, Suzuki S, Tachimori S (2001) The novel extractants, diglycolamides, for the extraction of lanthanides and actindes in HNO3-n-dodecane system. Solv Extr Ion Exch 19:91–103
Sasaki Y, Zhu Z, Sugo Y, Kimura T (2007) Extraction of various metal ions from nitric acid ton-dodecanen by diglycolamide (DGA) compounds. J Nucl Sci Technol 44:405–409
Ansari SA, Pathak P, Mohapatra PK, Manchanda VK (2012) Chemistry of diglycolamides: promising extractants for actinide partitioning. Chem Rev 112:1751–1772
Mowafy EA, Mohamed D (2015) Extraction and separation of Nd(III), Sm(III), Dy(III), Fe(III), Ni(II), and Cs(I) from concentrated chloride solutions with N, N, N′, N′-tetra(2-ethylhexyl) diglycolamide as new extractant. J Rare Earths 33:432–438
Mowafy EA, Mohamed D (2017) Extraction of rare earth elements from nitrate solution using novel unsymmetrical diglycolamide. Sep Sci Technol 52:1006–1014
Sasaki Y, Sugo Y, Kitatsuji Y, Kirishima A, Kimura T, Choppin GR (2007) Complexation and back extraction of various metals by water-soluble diglycolamide. Anal Sci 23:727–731
Sasaki Y, Suzuki H, Sugo Y, Kimura T, Choppin GR (2006) New water-soluble organic ligands for actinide cations complexation. Chem Lett 35:256–257
Sasaki Y, Sugo Y, Morita K, Nash KL (2015) The effect of alkyl substituents on actinide and lanthanide extraction by diglycolamide compounds. Solv Extr Ion Exch 33:625–641
Shen Y, Tan X, Wang L, Wu W (2011) Extraction of the uranyl ion from the aqueous phase into an ionic liquid by diglycolamide. Sep Purif Technol 78:298–302
Magnusson D, Christiansen B, Glatz JP, Malmbeck R, Modolo G, Serrano-Purroy D, Sorel C (2009) Demonstration of a TODGA based extraction process for the partitioning of minor actinides from a PUREX raffinate part III: centrifugal contactor run using genuine fuel solution. Solv Extr Ion Exch 27:26–35
Modolo G, Asp H, Vijgen H, Malmbeck R, Magnusson D, Sorel C (2008) Demonstration of a TODGA-based continuous counter-current extraction process for the partitioning of actinides from a simulated PUREX raffinate, part II: centrifugal contactor runs. Solv Extr Ion Exch 26:62–76
Modolo G, Asp H, Schreinemachers C, Vijgen H (2007) Development of a TODGA based process for partitioning of actinides from a PUREX raffinate part I: batch extraction optimization studies and stability tests. Solv Extr Ion Exch 25:703–721
Gujar RB, Ansari SA, Prabhu DR, Raut DR, Pathak PN, Sengupta A, Thulasidas SK, Mohapatra PK, Manchanda VK (2010) Demonstration of T2EHDGA based process for actinide partitioning part II: counter-current extraction studies. Solv Extr Ion Exch 28:764–777
Gujar RB, Ansari SA, Mohapatra PK, Manchanda VK (2010) Development of T2EHDGA based process for actinide partitioning. Part I: batch studies for process optimization. Solv Extr Ion Exch 28:350–366
Ravi J, Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2013) Unsymmetrical diglycolamide for the safe management of nuclear waste. J Environ Chem Eng 1:690–695
Ravi J, Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2014) Feasibility of using di-dodecyl-di-octyl diglycolamide for partitioning of minor actinides from fast reactor high-level liquid waste. Solv Extr Ion Exch 32:424–436
Guoxin S, Min L, Yu C, Meilong Y, Shaohong Y (2010) Synthesis of N, N′-dimethyl-N, N′-dioctyl-3-oxadiglycolamide and its extraction properties for lanthanides. Solv Extr Ion Exch 28:482–494
Ravi J, Venkatesan KA, Antony MP, Srinivasan TG, Rao PRV (2013) Tuning the diglycolamides for modifier-free minor actinide partitioning. J Radioanal Nucl Chem 295:1283–1292
Ravi J, Suneesh AS, Prathibha T, Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2011) Extraction behavior of some actinides and fission products from nitric acid medium by a new unsymmetrical diglycolamide. Solv Extr Ion Exch 29:86–105
Sasaki Y, Ozawa M, Kimura T, Ohashi K (2009) 2,2′-(Methylimino)bis(N, N-dioctylacetamide) (MIDOA), a new tridentate extractant for technetium(VII), rhenium(VII), palladium(II), and plutonium(IV). Solv Extr Ion Exch 27:378–394
Kim E, Boulègue J (2003) Chemistry of rhenium as an analogue of technetium: experimental studies of the dissolution of rhenium oxides in aqueous solutions. Radiochim Acta 91:211–216
Rouschias G (1974) Recent advances in the chemistry of rhenium. Chem Rev 74:531–566
Sato T, Sato K (1990) Liquid–liquid extraction of rhenium (VII) from hydrochloric acid solutions by neutral organophosphorus compounds and high molecular weight amines. Hydrometallurgy 25:281–291
Xiong Y, Lou Z, Yue S, Song J, Shan W, Han G (2010) Kinetics and mechanism of Re(VII) extraction with mixtures of tri-alkylamine and tri-n-butylphosphate. Hydrometallurgy 96:110–115
Srivastava RR, Kim M, Lee J, Ilyas S (2015) Liquid–liquid extraction of rhenium(VII) from an acidic chloride solution using Cyanex 923. Hydrometallurgy 157:33–38
Ogata T, Takeshita K, Fugate GA, Mori A (2008) Extraction of soft metals from acidic media with nitrogen-donor ligand TPEN and its analogs. Sep Sci Technol 43:2630–2640
Zhu Z, Sasaki Y, Suzuki H, Suzuki S, Kimura T (2004) Cumulative study on solvent extraction of elements by N,N,N′,N′-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into n-dodecane. Anal Chim Acta 527:163–168
Huang Z-W, Li Z-J, Wu Q-Y, Zheng L-R, Zhou L-M, Chai Z-F, Wang X-L, Shi W-Q (2018) Simultaneous elimination of cationic uranium(vi) and anionic rhenium(vii) by graphene oxide–poly(ethyleneimine) macrostructures: a batch, XPS, EXAFS, and DFT combined study. Environ Sci Nano 5:2077–2087
Wang S, Yu P, Purse BA, Orta MJ, Diwu J, Casey WH, Phillips BL, Alekseev EV, Depmeier W, Hobbs DT, Albrecht-Schmitt TE (2012) Selectivity, kinetics, and efficiency of reversible anion exchange with TcO4− in a supertetrahedral cationic framework. Adv Funct Mater 22:2241–2250
Helz GR, Dolor MK (2012) What regulates rhenium deposition in euxinic basins? Chem Geol 304–305:131–141
Stepinski DC, Vandegrift GF, Shkrob IA, Wishart JF, Kerr K, Dietz ML, Qadah DTD, Garvey SL (2010) Extraction of tetra-oxo anions into a hydrophobic, ionic liquid-based solvent without concomitant ion exchange. Ind Eng Chem Res 49:5863–5868
Xu Y, Gao Y, Zhou Y, Fan C, Hou H, Zhang M (2017) Extraction behavior of strontium from nitric acid medium with N,N′-dimethyl-N,N′-dioctyldiglcolamide. Solv Extr Ion Exch 35:507–518
Srivastava RR, Lee J, Kim M (2015) Complexation chemistry in liquid–liquid extraction of rhenium. J Chem Technol Biotechnol 90:1752–1764
Liu YY, Gao Y, Wei Z, Zhou Y, Zhang M, Hou HG, Tian GX, He H (2018) Extraction behavior and third phase formation of neodymium(III) from nitric acid medium in N,N′-dimethyl-N,N′-dioctyl-3-oxadiglcolamide. J Radioanal Nucl Chem 318:2087–2096
Ali MC, Kawasaki T, Nogami M, Saeki M, Sasaki Y, Iked Y (2011) selective extraction of perrhenate anion in nitric acid solution using 2,2′-(imino)bis(N,N′-dioctylacetamide) as an extractant. Prog Nucl Energy 53:1005–1008
Sasaki Y, Morita K, Shimazaki S, Tsubata Y, Ozawa M (2016) Masking effects for Mo, Re, Pd and Ru by S and N-donor reagents through MIDOA and NTAamide extraction. Solv Extr Res Dev 23:161–174
Sasaki Y, Saeki M, Yoshizuka K (2019) Extractions and spectroscopic studies of various metals with diglycolamide-type tridentate ligands. Solv Extr Res Dev 26:21–34
Pearson RG (1968) Hard and soft acids and bases, HSAB, part 1: fundamental principles. J Chem Educ 45:581
Cai XC, Cui Y, Yang XF, Li YX, Sun GX (2016) Extraction of U(VI) from nitric acid medium by N,N,N’,N’-tetra-2-ethylhexyldiglycolamide in sulfonated kerosene. Chin J Inorg Chem 32:1089–1094
Ren P, Yue Y-Z, Wang K, Wu W-S, Yan Z-Y (2014) Synthesis and characterization of N,N,N′,N′-tetraalkyl-4-oxaheptanediamide as extractant for extraction of uranium(VI) and thorium(IV) ions from nitric acid solution. J Radioanal Nucl Chem 300:1099–1103
Tian G, Zhang P, Wang J, Rao L (2005) Extraction of actinide(III, IV, V, VI) ions and TcO4− by N,N,N′,N′-tetraisobutyl-3-oxa-glutaramide. Solv Extr Ion Exch 23:631–643
Stephan H, Gloe K, Beger J, Mühl P (1991) Liquid–liquid extraction of metal ions with amido podands. Solv Extr Ion Exch 9:459–469
Panja S, Mohapatra PK, Tripathi SC, Manchanda VK (2011) Facilitated transport of uranium(VI) across supported liquid membranes containing T2EHDGA as the carrier extractant. J Hazard Mater 188:281–287
Sasaki Y, Morita Y, Kitatsuji Y, Kimura T (2010) Extraction behavior of actinides and metal ions by the promising extractant, N,N,N′,N′-tetraoctyl-3,6-dioxaoctanediamide (DOODA). Solv Extr Ion Exch 28:335–349
Favre-Réguillon A, Draye M, Cote G, Czerwinsky KR (2019) Insights in uranium extraction from spent nuclear fuels using dicyclohexano-18-crown-6—fate of rhenium as technetium homolog. Sep Purif Technol 209:338–342
Buhl M, Golubnychiy V (2007) Binding of pertechnetate to uranyl(VI) in aqueous solution. A density functional theory molecular dynamics study. Inorg Chem 46:8129–8131
Sutton AD, John GH, Sarsfield MJ, Renshaw JC, May I, Martin LR, Selvage AJ, Collison D, Helliwell M (2004) Spectroscopic evidence for the direct coordination of the pertechnetate anion to the uranyl cation in [UO2(TcO4)(DPPMO2)2]+. Inorg Chem 43:5480–5482
Lin C (2012) Process chemistry of fission products elements. Process chemistry of Tc. China Atomic Energy Press, Beijing
Acknowledgements
The work was supported by the Special Fund of Central University Basic Scientific Research Fee under Grant (GK2150260176).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, Z., Lu, C., Zhou, Y. et al. Extraction and separation performance of rhenium(VII) and uranium(VI) from nitric acid medium using N,N′-dimethyl-N,N′-dioctyldiglycolamide. J Radioanal Nucl Chem 323, 875–884 (2020). https://doi.org/10.1007/s10967-019-06976-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06976-x