Abstract
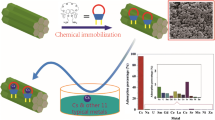
As an effective cesium complex agent, calix[4]biscrown-6 (CBC) applied at a low cost way is of interest. In this study, CBC/XAD-7 was prepared by embedding CBC into XAD-7. Subsequently the as-prepared sorbent was used for the removal of cesium from aqueous solution as functions of HNO3 concentration, contact time, temperature and initial cesium concentration. The results revealed that the nitric concentration influenced cesium adsorption by complex and protonation interaction. The most effective adsorption happened at the nitric concentration of 1.0 M. The adsorption isotherm well described with the Langmuir model illustrated a monolayer adsorption. Its maximum adsorption capacity was 24.4 mg/g in the 2 M nitric acid aqueous solution. The adsorption kinetics was in accordance with the pseudo-second order model, which indicated a chemisorption. The thermodynamic parameters demonstrated that the adsorption process was exothermal and spontaneous. In addition CBC/XAD-7 showed highly selective recognition toward cesium and good reusability. The study offered an economical and effective material for cesium removal.







Similar content being viewed by others
References
Berndes G, Hoogwijk M, Van den Broek R (2003) The contribution of biomass in the future global energy supply: a review of 17 studies. Biomass Bioenergy 25(1):1–28
Bilgen S (2014) Structure and environmental impact of global energy consumption. Renew Sustain Energy Rev 38:890–902
Breyer C, Bogdanov D, Aghahosseini A et al (2018) Solar photovoltaics demand for the global energy transition in the power sector. Prog Photovolt Res Appl 26(8):505–523
Kesieme U, Chrysanthou A, Catulli M et al (2018) A review of acid recovery from acidic mining waste solutions using solvent extraction. J Chem Technol Biotechnol 93(12):3374–3385
Wu Y, Lee CP, Mimura H et al (2018) Stable solidification of silica-based ammonium molybdophosphate by allophane: application to treatment of radioactive cesium in secondary solid wastes generated from fukushima. J Hazard Mater 341:46–54
Swami KR, Prathibha T, Venkatesan KA et al (2019) Separation of Am (III) from Eu (III) present in 3 M nitric acid medium using completely incinerable binary extractants. J Mol Liq. 279:427–433
Figueiredo BR, Cardoso SP, Portugal I et al (2018) Inorganic ion exchangers for cesium removal from radioactive wastewater. Sep Purif Rev 47(4):306–336
Tanaka S, Adati T, Takahashi T et al (2018) Concentrations and biological half-life of radioactive cesium in epigeic earthworms after the Fukushima Dai-ichi Nuclear Power Plant accident. J Environ Radioact 192:227–232
Vipin AK, Fugetsu B, Sakata I et al (2016) Cellulose nanofiber backboned Prussian blue nanoparticles as powerful adsorbents for the selective elimination of radioactive cesium. Sci Rep 6:37009
Zhang H, Tangparitkul S, Hendry B et al (2019) Selective separation of cesium contaminated clays from pristine clays by flotation. Chem Eng J 355:797–804
Wang K, Ma H, Pu S et al (2019) Hybrid porous magnetic bentonite-chitosan beads for selective removal of radioactive cesium in water. J Hazard Mater 362:160–169
Husnain SM, Um W, Chang YY et al (2017) Recyclable superparamagnetic adsorbent based on mesoporous carbon for sequestration of radioactive Cesium. Chem Eng J 308:798–808
Lonin AY, Levenets VV, Neklyudov IM et al (2015) The usage of zeolites for dynamic sorption of cesium from waste waters of nuclear power plants. J Radioanal Nucl Chem 303(1):831–836
Bengiat R, Bogoslavsky B, Mandler D et al (2018) Selective binding and precipitation of cesium ions from aqueous solutions: a size-driven supramolecular reaction. Chem Eur J 24(13):3161–3164
Li Z, Pranolo Y, Zhu Z et al (2017) Solvent extraction of cesium and rubidium from brine solutions using 4-tert-butyl-2-(α-methylbenzyl)-phenol. Hydrometallurgy 171:1–7
Chakravarty R, Ram R, Pillai KT et al (2012) Ammonium molybdophosphate impregnated alumina microspheres as a new generation sorbent for chromatographic 137Cs/137mBa generator. J Chromatogr A 1220:82–91
Kim H, Kim M, Kim W et al (2018) Photocatalytic enhancement of cesium removal by Prussian blue-deposited TiO2. J Hazard Mater 357:449–456
Dai Y, Zhang A (2014) Extraction equilibrium and thermodynamics of cesium with a new derivative of calix[4]biscrown. J Radioanal Nucl Chem 302(1):575–581
Kumar N, Pham-Xuan Q, Depauw A et al (2017) New sensitive and selective calixarene-based fluorescent sensors for the detection of Cs+ in an organoaqueous medium. New J Chem 41(15):7162–7170
Xiao C, Zhang A (2016) Synthesis and characterization of a cesium-selective macroporous silica-based supramolecular recognition material with high stability. J Radioanal Nucl Chem 307(1):713–723
Yi R, Xu C, Sun T et al (2018) Improvement of the extraction ability of bis (2-propyloxy) calix[4]arene-crown-6 toward cesium cation by introducing an intramolecular triple cooperative effect. Sep Purif Technol 199:97–104
Depauw A, Kumar N, Ha-Thi MH et al (2015) Calixarene-based fluorescent sensors for cesium cations containing BODIPY fluorophore. J Phys Chem A 119(23):6065–6073
Urban I, Ratcliffe NM, Duffield JR et al (2010) Functionalized paramagnetic nanoparticles for waste water treatment. Chem Commun 46(25):4583–4585
Kumar R, Lee YO, Bhalla V et al (2014) Recent developments of thiacalixarene based molecular motifs. Chem Soc Rev 43(13):4824–4870
İnan S, Tel H, Sert Ş et al (2018) Extraction and separation studies of rare earth elements using Cyanex 272 impregnated Amberlite XAD-7 resin. Hydrometallurgy 181:156–163
Dai Y, Lv R, Liu Z et al (2018) Extraction behavior of cesium from nitric acid medium with calix [4]-bis [(4-tert-butyl-1, 2-phenylene)-crown-6]. J Radioanal Nucl Chem 318(3):2079–2086
Tao Q, Wang X, Huang D et al (2018) Adsorption of cesium from aqueous solution of highly concentrated nitric acid using supermolecule/ordered mesoporous carbon composite. Water Air Soil Pollut 229(11):361
Pan B, Zhang X, Jiang Z et al (2019) Polymer and polymer-based nanocomposite adsorbents for water treatment[M]//polymeric materials for clean water. Springer, Cham, pp 93–119
Long GL, Winefordner JD (1983) Limit of detection. A closer look at the IUPAC definition. Anal Chem 55(7):712–724
Zhang A, Chen C, Ji Y et al (2018) Uptake of cesium and some typical metals onto hybrid calix[4]crown adsorbent with silica carrier by host-guest recognition. J Chem Eng Data 63(5):1578–1587
Khandaker S, Toyohara Y, Kamida S et al (2018) Effective removal of cesium from wastewater solutions using an innovative low-cost adsorbent developed from sewage sludge molten slag[J]. J Environ Manag 222:304–315
Mashhadi S, Javadian H, Ghasemi M et al (2016) Microwave-induced H2SO4 activation of activated carbon derived from rice agricultural wastes for sorption of methylene blue from aqueous solution. Desalin Water Treat 57(44):21091–21104
Hosseini-Bandegharaei A, Allahabadi A, Rahmani-Sani A et al (2016) Thorium removal from weakly acidic solutions using titan yellow-impregnated XAD-7 resin beads: kinetics, equilibrium and thermodynamic studies. J Radioanal Nucl Chem 309(2):761–776
Deng H, Li Y, Huang Y et al (2016) An efficient composite ion exchanger of silica matrix impregnated with ammonium molybdophosphate for cesium uptake from aqueous solution[J]. Chem Eng J 286:25–35
Kang K, Lohrman JA, Nagarajan S et al (2019) Convergent ditopic receptors enhance anion binding upon alkali metal complexation for catalyzing the ritter reaction. Organic Lett. 21(3):652–655
El-Latif MMA, Elkady MF (2011) Kinetics study and thermodynamic behavior for removing cesium, cobalt and nickel ions from aqueous solution using nano-zirconium vanadate ion exchanger. Desalination 271(1–3):41–54
Hassan NM, Adu-Wusu K (2005) Cesium removal from Hanford tank waste solution using resorcinol-formaldehyde resin. Solvent Extr Ion Exch 23(3):375–389
Miah MY, Volchek K, Kuang W et al (2010) Kinetic and equilibrium studies of cesium adsorption on ceiling tiles from aqueous solutions. J Hazard Mater 183(1–3):712–717
Hadadi N, Kananpanah S, Abolghasemi H (2009) Equilibrium and thermodynamic studies of cesium adsorption on natural vermiculite and optimization of operation conditions. Iran J Chem Chem Eng (IJCCE) 28(4):29–36
Negrea A, Lupa L, Ciopec M, et al. (2014) Silica impregnated with cyphos IL-101 for Cs+ adsorption. Environ Eng Manag J (EEMJ) 13(8):2005–2013
Olatunji MA, Khandaker MU, Mahmud EHNM et al (2018) Remediation of 137 Cs radionuclide in nuclear waste effluents by polymer composite: adsorption kinetics, isotherms and gamma irradiation studies. J Radioanal Nucl Chem 316:933–945
Zheng X, Dou J, Yuan J et al (2017) Removal of Cs+ from water and soil by ammonium-pillared montmorillonite/Fe3O4 composite. J Environ Sci 56:12–24
Alamudy HA, Cho K (2018) Selective adsorption of cesium from an aqueous solution by a montmorillonite-prussian blue hybrid. Chem Eng J 349:595–602
Ding D, Zhao Y, Yang S et al (2013) Adsorption of cesium from aqueous solution using agricultural residue–walnut shell: equilibrium, kinetic and thermodynamic modeling studies. Water Res 47(7):2563–2571
Acknowledgements
The present work was financially supported by National Natural Science Foundation of China (No. 11605027, 11705060), the Natural Science Foundation of Jiangxi Province (No. 20161BAB213086), and the Opening Project of Jiangxi Province Key Laboratory of Polymer Micro/Nano Manufacturing and Devices.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, Y., Lv, R., Fan, J. et al. Adsorption of cesium using supermolecular impregnated XAD-7 composite: isotherms, kinetics and thermodynamics. J Radioanal Nucl Chem 321, 473–480 (2019). https://doi.org/10.1007/s10967-019-06625-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06625-3