Abstract
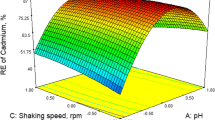
In this study, the removal of hexavalent chromium, a nonradioactive analogue of pertechnetate, from aqueous solution by bamboo shoot shell (BSS) biosorbent was investigated. Characterization of the sorbent was performed by element analysis and Fourier transform infrared spectroscopy. The effects of diverse parameters including solution pH, adsorbent concentration and adsorption time on the adsorption process were discussed in detail. The adsorption of Cr(VI) by the BSS obeyed Elovich kinetic model and Freundlich isotherm model. The maximum adsorption capacity based on Langmuir model for BSS was 28.72 mg/g, which was higher than that of other biosorbents previously reported in the literature. The thermodynamic analysis suggested that Cr(VI) adsorption was a spontaneous endothermic process. On the basis of these results, an adsorption-reduction interaction on the heterogeneous surface of biosorbent would be the predominant mechanism. The findings herein revealed that the BSS had great potential as a suitable and sustainable biosorbent material for the removal of pertechnetate.





Similar content being viewed by others
References
Gagnon K, Benard F, Kovacs M, Ruth TJ, Schaffer P, Wilson JS, McQuarrie SA (2011) Cyclotron production of 99mTc: experimental measurement of the 100Mo(p, x)99Mo, 99mTc and 99gTc excitation functions from 8 to 18 MeV. Nucl Med Biol 38(6):907–916
Liu S (2007) Ether and crown ether-containing cationic 99mTc complexes useful as radiopharmaceuticals for heart imaging. Dalton Trans 12:1183–1193
Hock SJ, Schaper LA, Herrmann WA, Kuhn FE (2013) Group 7 transition metal complexes with N-heterocyclic carbenes. Chem Soc Rev 42(12):5073–5089
Liu S (2008) Bifunctional coupling agents for radiolabeling of biornolecules and target-specific delivery of metallic radionuclides. Adv Drug Deliv Rev 60(12):1347–1370
Villar M, Borras A, Avivar J, Vega F, Cerda V, Ferrer L (2017) Fully automated system for 99Tc monitoring in hospital and urban residues: a simple approach to waste management. Anal Chem 89(11):5858–5864
Krawczyk E, Pinero-Garcia F, Ferro-Garcia MA (2013) Discharges of nuclear medicine radioisotopes in Spanish hospitals. J Environ Radioact 116:93–98
Markovic BM, Jankovic DL, Vukadinovic AA, Randelovic DV, Maksin DD, Spasojevic VV, Nastasovic AB (2017) A novel macroporous polymer-inorganic nanocomposite as a sorbent for pertechnetate ions. RSC Adv 7(35):21412–21421
Seliman AF, Helariutta K, Wiktorowicz SJ, Tenhu H, Harjula R (2013) Stable and selective scintillating anion-exchange sensors for quantification of 99TcO4 − in natural freshwaters. J Environ Radioact 126:156–164
Bennett R, Willey N (2003) Soil availability, plant uptake and soil to plant transfer of 99Tc—a review. J Environ Radioact 65(2):215–231
Zachara JM, Heald SM, Jeon B-H, Kukkadapu RK, Liu C, McKinley JP, Dohnalkova AC, Moore DA (2007) Reduction of pertechnetate Tc(VII) by aqueous Fe(II) and the nature of solid phase redox products. Geochim Cosmochim Acta 71(9):2137–2157
Volesky B (2007) Biosorption and me. Water Res 41(18):4017–4029
Hu H, Jiang B, Wu H, Zhang J, Chen X (2016) Bamboo (Acidosasa edulis) shoot shell biochar: its potential isolation and mechanism to perrhenate as a chemical surrogate for pertechnetate. J Environ Radioact 165:39–46
Shu X, Shen L, Wei Y, Hua D (2015) Synthesis of surface ion-imprinted magnetic microsphere for efficient sorption of perrhenate: a structural surrogate for pertechnetate. J Mol Liq 211:621–627
Buechele AC, McKeown DA, Lukens WW, Shuh DK, Pegg IL (2012) Tc and Re behavior in borosilicate waste glass vapor hydration tests II. J Nucl Mater 429(1–3):159–165
Saslow SK, Um W, Pearce CI, Engelhard MH, Bowden ME, Lukens W, Leavy II, Riley BJ, Kirn DS, Schweiger MJ, Kruger AA (2017) Reduction and simultaneous removal of 99Tc and Cr by Fe(OH)2(s) mineral transformation. Environ Sci Technol 51(15):8635–8642
Meena AH, Kaplan DI, Powell BA, Arai Y (2015) Chemical stabilization of chromate in blast furnace slag mixed cementitious materials. Chemosphere 138:247–252
Saha B, Orvig C (2010) Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord Chem Rev 254(23):2959–2972
Inyang MI, Gao B, Yao Y, Xue Y, Zimmerman A, Mosa A, Pullammanappallil P, Yong SO, Cao X (2016) A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit Rev Environ Sci Technol 46(4):406–433
Venugopal V, Mohanty K (2011) Biosorptive uptake of Cr(VI) from aqueous solutions by Parthenium hysterophorus weed: equilibrium, kinetics and thermodynamic studies. Chem Eng J 174(1):151–158
Singha B, Das SK (2013) Adsorptive removal of Cu(II) from aqueous solution and industrial effluent using natural/agricultural wastes. Colloid Surf B 107(4):97–106
Kul AR, Koyuncu H (2010) Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: kinetic, equilibrium and thermodynamic study. J Hazard Mater 179(1):332–339
Prabhakaran SK, Vijayaraghavan K, Balasubramanian R (2009) Removal of Cr(VI) Ions by spent tea and coffee dusts: reduction to Cr(III) and biosorption. Ind Eng Chem Res 48(4):2113–2117
Lu W, Guo Y, Zhang B, Wang C (2013) Comprehensive analysis on elements, energy recovery, and oil compositions of biomass deoxy-liquefaction. Energy Fuel 27(4):2157–2166
Wang C, Pan J, Li J, Yang Z (2008) Comparative studies of products produced from four different biomass samples via deoxy-liquefaction. Bioresour Technol 99(8):2778–2786
Blanes PS, Bordoni ME, González JC, García SI, Atria AM, Sala LF, Bellú SE (2016) Application of soy hull biomass in removal of Cr(VI) from contaminated waters. Kinetic, thermodynamic and continuous sorption studies. J Environ Chem Eng 4(1):516–526
Al-Wabel MI, Al-Omran A, El-Naggar AH, Nadeem M, Usman ARA (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour Technol 131(3):374–379
Torab-Mostaedi M, Asadollahzadeh M, Hemmati A, Khosravi A (2013) Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grapefruit peel. J Taiwan Inst Chem Eng 44(2):295–302
Srivastava S, Agrawal SB, Mondal MK (2015) Biosorption isotherms and kinetics on removal of Cr(VI) using native and chemically modified Lagerstroemia speciosa bark. Ecol Eng 85(3):56–66
Shen YS, Wang SL, Huang ST, Tzou YM, Huang JH (2010) Biosorption of Cr(VI) by coconut coir: spectroscopic investigation on the reaction mechanism of Cr(VI) with lignocellulosic material. J Hazard Mater 179(1):160–165
Panda GC, Das SK, Bandopadhyay TS, Guha AK (2007) Adsorption of nickel on husk of Lathyrus sativus: behavior and binding mechanism. Colloid Surf B 57(2):135–142
Hui H, Zhang J, Lu K, Tian Y (2015) Characterization of Acidosasa edulis shoot shell and its biosorption of copper ions from aqueous solution. J Environ Chem Eng 3(1):357–364
Iftikhar AR, Bhatti HN, Hanif MA, Nadeem R (2009) Kinetic and thermodynamic aspects of Cu(II) and Cr(III) removal from aqueous solutions using rose waste biomass. J Hazard Mater 161(2):941–947
Shi S, Yang J, Liang S, Li M, Gan Q, Xiao K, Hu J (2018) Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2–NH2 particles. Sci Total Environ 628–629:499–508
Zhou Y, Liu X, Tang L, Zhang F, Zeng G, Peng X, Luo L, Deng Y, Pang Y, Zhang J (2017) Insight into highly efficient co-removal of p-nitrophenol and lead by nitrogen-functionalized magnetic ordered mesoporous carbon: performance and modelling. J Hazard Mater 333:80–87
Xiong W, Tong J, Yang Z, Zeng G, Zhou Y, Wang D, Song P, Xu R, Zhang C, Cheng M (2017) Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: performance and mechanism. J Colloid Interface Sci 493:17–23
Park D, Yun Y-S, Kim JY, Park JM (2008) How to study Cr(VI) biosorption: use of fermentation waste for detoxifying Cr(VI) in aqueous solution. Chem Eng J 136(2–3):173–179
Drweesh SA, Fathy NA, Wahba MA, Hanna AA, Akarish AIM, Elzahany EAM, El-Sherif IY, Abou-El-Sherbini KS (2016) Equilibrium, kinetic and thermodynamic studies of Pb(II) adsorption from aqueous solutions on HCl-treated Egyptian kaolin. J Environ Chem Eng 4(2):1674–1684
Aguilar-Carrillo J, Garrido F, Barrios L, Garcia-Gonzalez MT (2006) Sorption of As, Cd and Tl as influenced by industrial by-products applied to an acidic soil: equilibrium and kinetic experiments. Chemosphere 65(11):2377–2387
Deng H, Yang L, Tao G, Dai J (2009) Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—application in methylene blue adsorption from aqueous solution. J Hazard Mater 166(2):1514–1521
Jang HM, Yoo S, Choi YK, Park S, Kan E (2018) Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour Technol 259:24–31
Singha B, Das SK (2011) Biosorption of Cr(VI) ions from aqueous solutions: kinetics, equilibrium, thermodynamics and desorption studies. Colloid Surf B 84(1):221–232
Elkady MF, Ibrahim AM, El-Latif MMA (2011) Assessment of the adsorption kinetics, equilibrium and thermodynamic for the potential removal of reactive red dye using eggshell biocomposite beads. Desalination 278(1):412–423
Li TT, Liu YG, Peng QQ, Hu LT, Hui W (2013) Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: kinetic and equilibrium modeling. Chem Eng J 214(4):189–197
Malamis S, Katsou E (2013) A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: examination of process parameters, kinetics and isotherms. J Hazard Mater 252–253(10):428–461
Deng L, Su Y, Su H, Wang X, Zhu X (2007) Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater 143(1):220–225
Liang S, Guo X, Lautner S, Saake B (2014) Removal of hexavalent chromium by different modified spruce bark adsorbents. J Wood Chem Technol 34(4):273–290
Gao H, Liu Y, Zeng G, Xu W, Li T, Xia W (2008) Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste-rice straw. J Hazard Mater 150(2):446–452
Munir K, Yusuf M, Noreen Z, Hameed A, Hafeez FY, Faryal R (2010) Isotherm studies for determination of removal capacity of bi-metal (Ni and Cr) ions by Aspergillus niger. Pak J Bot 42(1):593–604
Dakiky M, Khamis M, Manassra A, Mer’Eb M (2002) Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res 6(4):533–540
Gebrehawaria G, Hussen A, Rao VM (2015) Removal of hexavalent chromium from aqueous solutions using barks of Acacia albida and leaves of Euclea schimperi. Int J Environ Sci Technol 12(5):1569–1580
Šćiban MB, Klašnja MT, Antov MG (2011) Study of the biosorption of different heavy metal ions onto Kraft lignin. Ecol Eng 37(12):2092–2095
Dönmez GÇ, Aksu Z, Öztürk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34(9):885–892
Bhattacharya A, Naiya T, Mandal S, Das S (2007) Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J 137(2007):529–541
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, H., Gao, Y., Wang, T. et al. Removal of hexavalent chromium, an analogue of pertechnetate, from aqueous solution using bamboo (Acidosasa edulis) shoot shell. J Radioanal Nucl Chem 321, 427–437 (2019). https://doi.org/10.1007/s10967-019-06606-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06606-6