Abstract
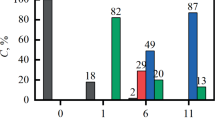
This study is aimed at developing a promising method of strontium impurity separation from concentrated molybdenum solutions originating from molybdenum recycling from irradiated CerMet nuclear fuels with an isotopically tailored molybdenum matrix. Strontium sorption onto thirteen inorganic or composite absorbers from a slightly alkaline (pH 9.1) ammonium molybdate surrogate solution was studied. Based on the evaluation of weight distribution ratios, their dependence on molybdenum concentration and pH, and kinetics of sorption, calcium activated barium sulfate (Ba(Ca)SO4) was identified as the most promising material. In a dynamic column experiment performed with the Ba(Ca)SO4-PAN absorber, 2700 BV of the solution with cSr = 10−4 mol L−1 could be treated with a breakthrough of lower than 1% and 100% breakthrough was not achieved even after processing almost 7000 BV of the feed.





Similar content being viewed by others
References
Mareš KV, John J (2019) Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: part 1—concept design and assessment. J Radioanal Nucl Chem 320(1):227–233. https://doi.org/10.1007/s10967-019-06456-2
Mareš KV, John J (2019) Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: part 2—caesium separation from concentrated molybdate solution. J Radioanal Nucl Chem 320(2):377-384. https://doi.org/10.1007/s10967-019-06480-2
Bakker K et al (2004) The use of molybdenum-based ceramic-metal (CerMet) Fuel for the actinide management in LWRs. Nucl Technol 146:325–331
Haas D et al (2006) Properties of CerMet fuels for minor actinides transmutation in ADS. Energy Convers Manag 47:2724–2731
Advanced fuelS for Generation IV reActors: Reprocessing and Dissolution—ASGARD (2012–2015) EURATOM FP7-Fission-2011 project No. 295825, http://asgardproject.eu/
Sebenik RF et al (2012) Molybdenum and molybdenum compounds. In: Bohnet M, Brinker CJ, Cornils B (eds) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, NewYork
Davis JA, Kent DB (1990) Surface complexation modeling in aqueous geochemistry. In: Hochella MF, White AF (eds) Mineral-water interface geochemistry, vol 23. Mineralogical Society of America, Washington, pp 177–260
Moon DS, Burnett WC, Nour S, Horwity P, Bond A (2003) Preconcentration of radium isotopes from natural waters using MnO2 resin. Appl Radiat Isot 59:255–262
Anthony RG, Philip CV, Dosch RG (1993) Selective adsorption and ion exchange of metal cations and anions with silico-titanates and layered titanates. Waste Manag 13:503–512
Fryxell GE, Cao G (eds) (2012) Environmental applications of nanomaterials-synthesis, sorbents and sensors, 2nd edn. Imperial College Press, London
Klavetter EA, Brown NE, Trudell D, Zheng Z, Thibaud-Erhey C, Ding G, Rayford GA (1994) Performance of crystalline silicotitanates for cesium removal from Hanford tank waste simulants. SAN094-2380C
Miller JE, Brown NE (1997) Development and properties of crystalline silicotitanate (CST) ion exchangers for radioactive waste applications. Sandia National Laboratories, SAND97-0771
Lehto J (1987) Sodium titanate for solidification of radioactive wastes—preparation, structure and ion exchange properties. Academic Dissertation. Report Series in Radiochemistry 5/1987, Univestity of Helsinki, p 38
Lehto J, Clearfield A (1987) The ion exchange of strontium on sodium titane Na4Ti9O20·xH2O. J Radioanal Nucl Chem Lett 118:1–13
Rosskopfová O, Galamboš M, Rajec P (2011) Study of sorption processes of strontium on the synthetic hydroxyapatite. J Radioanal Nucl Chem 287:715–722
IAEA Technical Reports Series No 236 (1984), Vienna
Málková E (2016) Synthesis and study on nanocarriers for nuclear medicine (in Czech), Diploma thesis, CTU in Prague—FNSPE
Berák L (1965) Activated barium sulphate as a sorbent of small quantities of strontium. Collect Czechoslov Chem Commun 30(5):1490–1504
Berák L (1961) Czechoslovak Patent No. 102 881
Berák L, Münich J (1966) Barium sulfate activated for sorption of strontium by heat treatment with calcium sulfate. Collect Czechoslov Chem Commun 31:881–901
Manos MJ, Ding N, Kanatyudis MG (2008) Layered metal sulfides: exceptionally selective agents for radioactive strontium removal. PNAS Natl Acad Sci USA 105(10):3696–3699
Collins JL, Egan BZ, Anderson KK, Chase CW, Bell JT (1995) Batch test equilibration studies examining the removal of Cs, Sr, and Tc from supernatants from ORNL underground storage tanks by selected ion exchangers. In: Proceedings of the international conference of waste management CONF-9505101–1
Šebesta F (1997) Composite absorbers consisting of inorganic ion-exchangers and polyacrylonitrile binding matrix. J Radioanal Nucl Chem 220(1):65–67
Šebesta F (1992) Czech Patent A. O. 273369
Šebesta F, John J, Motl A (1997) Removal of cesium and strontium from highly saline acidic or alkaline HLW using PAN-based composite absorbers. In: ICEM’97. American Society of Mechanical Engineers—ASME, New York, pp 241–244. ISBN 0-7918-1242-1
Šebesta F, John J, Motl A (1996) Removal of radium and thorium from water and saline solution using inorganic-organic composite absorbers with polyacrylonitrile binding matrix. In: Proceedings of IEX’96, pp 346–354
UOP A Honeywell Company (2012) UOP IONSIVTM ion exchangers: a superior nuclear waste remediation product. UOP A Honeywell Company. UOP5649a, USA
IBC Advanced Technologies: AnaLig® Data Sheet: Sr-01. IBC Advanced Technologies, Inc 15I102
Triskem: SR RESIN (Resins and Accessories): Sr resin Data Sheet http://www.triskem-international.com/catalog/products/resins-and-accessories/sr-resin/bl,product,412,0. Accessed 5 Feb 2019
Acknowledgements
This study was supported by the EU 7th Framework Programme project ASGARD (EC-GA No. 295825) focusing on research into advanced/novel fuels fabrication and their reprocessing issues for Generation IV reactors, by the Grant Agency of the Czech Technical University in Prague (Grants Nos. SGS12/199/OHK4/3T/14 and SGS15/216/OHK4/3T/14), and by the Centre for advanced applied science, Project Number CZ.02.1.01/0.0/0.0/16_019/0000778, supported by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mareš, K.V., Šebesta, F. & John, J. Recycling of isotopically modified molybdenum from irradiated CerMet nuclear fuel: part 3—strontium separation from concentrated molybdate solution. J Radioanal Nucl Chem 321, 277–284 (2019). https://doi.org/10.1007/s10967-019-06553-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06553-2