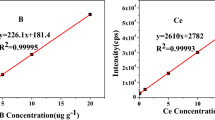
Abstract
Measurement methods for the analysis of trace impurities in uranium materials, essential in nuclear fuel production and nuclear forensics, are continuously improving. Analytical methods were developed with the goal of lowering uncertainties of next generation certified uranium oxide reference materials for trace impurity concentrations. Through addition of a traceable standard directly into the sample, gravimetric standard addition and isotope dilution followed by analysis on an inductively coupled plasma mass spectrometer can achieve lower uncertainties. Results for 28 impurities in CRM 124-1 and 124-6 from NBL Program Office were used for validation of accuracy and comparisons of uncertainties.






Similar content being viewed by others
Notes
The actual scope of the analysis was 28 elements, 17 by IDMS and 11 by gravimetric standard addition; however, 11 of those elements are not certified values in the CRM 124 series from NBLPO but are included for informational purposes.
The NIDC manages isotope production funded by the Isotope Development and Production for Research and Applications within the Office of Nuclear Physics of the United States Department of Energy Office of Science.
References
ASTM International (2016) Standard specification for nuclear-grade, sinterable uranium dioxide powder. ASTM C753-16a
ASTM International (2015) Standard specification for uranium hexafluoride for enrichment. ASTM C787-15
ASTM International (2015) Standard specification for nuclear-grade uranyl nitrate solution or crystals. ASTM C788-03
ASTM International (2017) Standard specification for sintered (uranium–plutonium) dioxide pellets for light water reactors. ASTM C833-17
ASTM International (2013) Standard specification for uranium ore concentrate. ASTM C967-13
ASTM International (2016) Standard specification for uranium oxides with a 235U content of less than 5% for dissolution prior to conversion to nuclear-grade uranium dioxide. ASTM C1334-05
Moody KJ, Hutcheon ID, Grant PM (2005) Nuclear forensic analysis. CRC Press, Boca Raton
Varga Z, Wallenius M, Mayer K, Meppen M (2011) Analysis of uranium ore concentrates for origin assessment. Proc Radiochim Acta 1:1–4
Keegan E, Wallenius M, Mayer K, Varga Z, Rasmussen G (2012) Attribution of uranium ore concentrates using elemental and anionic data. Appl Geochem 27:1600–1609
Varga Z, Krajkó J, Peńkin M, Novák M, Eke Z, Wallenius M, Mayer K (2017) Identification of uranium signatures relevant for nuclear safeguards and forensics. J Radioanal Nucl Chem 312:639–654
Inn KGW, Johnson CM Jr, Oldham W, Jerome S, Tandon L, Schaaff T, Jones R, Mackney D, MacKill P, Palmer B, Smith D, LaMont S, Griggs J (2012) The urgent requirement for new radioanalytical certified reference materials for nuclear safeguards, forensics, and consequence management. J Radioanal Nucl Chem 296:5–22
New Brunswick Laboratory (1983) Provisional certificate of analysis, CRM No. 124(1-7)
Bürger S, Mathew KJ, Mason P, Narayanan U (2009) Reference materials characterized for impurities in uranium matrices: an overview and re-evaluation of the NBL CRM 124 series. J Radioanal Nucl Chem 279(2):659–673
Mahan C, Bonchin S, Figg D, Gerth D, Collier C (2000) Chromatographic extraction of plutonium and inorganic impurity analysis using ICP-MS and ICP-AES. J Anal At Spectrom 15:929–935
Montoa DP, Manard BT, Xu N (2016) Novel sample introduction system to reduce ICP-OES sample size for plutonium metal trace impurity determination. J Radioanal Nucl Chem 307:2009–2014
Satyanarayana K, Durani S (2010) Separation and inductively coupled plasma optical emission spectrometric (ICP-OES) determination of trace impuriites in nuclear grade uranium oxide. J Radioanal Nucl Chem 285:659–665
Wylie E, Manard BT, Quarles CD Jr, Meyers L, Xu N (2018) An automated micro-separation system for the chromatographic removal of uranium matrix for trace element analysis by ICP-OES. Talanta. https://doi.org/10.1016/j.talanta.2018.06.063
Oliveira Junior OP, Sarkis JES (2002) Determination of impurities in uranium oxide by inductively coupled plasma mass spectrometry (ICPMS) by the matrix matching method. J Radioanal Nucl Chem 254(3):519–526
ASTM International (2010) Standard test method for determination of impurities in nuclear grade uranium compounds by inductively coupled plasma mass spectrometry. ASTM C1287-10
International Organization for Standardization (2010) Nuclear technology—nuclear fuels—procedures for the measurement of elemental impurities in uranium- and plutonium-based materials by inductively coupled plasma mass spectrometry, ISO 26062:2010(E)
Fassett JD (1988) Inorganic trace analysis by isotope dilution mass spectrometry—new frontiers. J Res Nat Bur Stand 93:417–418
Sargent M, Harrington C, Harte R (2002) Guidelines for achieveing high accuracy in isotope dilution mass spectrometry (IDMS). The Royal Society of Chemistry, Cambridge
Kelly WR, MacDonald BS, Guthrie WF (2008) Gravimetric approach to the standard addition method in instrumental analysis. 1. Anal Chem 80:6154–6158
Hauswaldt AL, Rienitz O, Jährling R, Fischer N, Schiel D, Labarraque G, Magnusson B (2012) Uncertainty of standard addition experiments: a novel approach to include the uncertainty associated with the standard in the model equation. Accred Qual Assur 17:129–138
ASTM International (2013) Standard practice for removal of uranium or plutonium, or both, for impurity assay in uranium or plutonium materials. ASTM C1647-13
Boulyga SF, Cunningham JA, Macsik Z, Hiess J, Peńkin MV, Walsh SJ (2017) Development. Validation and verification of an ICP-MS procedure for a multi-element analysis of uranium ore concentrates 32:2226–2237
Analytical Methods Committee, AMCTB No. 74 (2016) z-Scores and other scores in chemical proficiency testing—their meanings, and some common misconceptions. Anal Methods 8:5553–5555
Acknowledgements
Radioactive work requires many controls to ensure personnel and environment safety, and for that we thank Lisa Duncan, Mike Hensley and Micah Ely. The authors would like to thank Jeff Morrison as the program manager. This work is supported by the the Department of Homeland Security at the Department of Energy’s National Nuclear Security Administration under contract DE-AC05-00OR22725 with UT-Battelle, LLC. Oak Ridge National Laboratory is managed by UT-Battelle for the Department of Energy under Contract DE-AC05-000R22725. The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rogers, K.T., Giaquinto, J., Essex, R.M. et al. Trace impurity analysis in uranium oxide via hybrid quantification techniques—gravimetric standard addition and isotope dilution mass spectrometry. J Radioanal Nucl Chem 318, 685–694 (2018). https://doi.org/10.1007/s10967-018-6106-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6106-8