Abstract
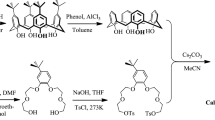
To separate heat-generating Cs from high-level liquid waste (HLLW), the extraction of Cs(I) using 1,3-[(2,4-diethylheptylethoxy)oxy]-2,4-crown-6-calix[4]arene (calix[4]arene-R14) in combination with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide) ionic liquids ([C n mim][NTf2], where n = 2, 4, and 6) was investigated. Under moderately acidic conditions, the calix[4]arene-R14/[C n mim][NTf2] system showed a higher extraction efficiency (ECs) than that exhibited by the conventional calix[4]arene-R14/dichloromethane system. All extraction processes reached equilibrium within 5 min. Slope analysis revealed that the Cs:calix[4]arene-R14 inclusion complex has a 1:1 stoichiometry. The extraction was found to be exothermic and influenced by the presence of Na(I). These extraction systems exhibited high efficiency and selectivity for Cs(I) in simulated HLLW.








Similar content being viewed by others
References
Ando Y, Takano H (1999) Estimation of LWR spent fuel composition. JAERI-Research 99-004
Mimura H, Kanno T (1985) Distribution and fixation of cesium and strontium in zeolite A and chabazite. J Nucl Sci Technol 22:284–291
McDowell JD, Case GN (1992) Selective extraction of cesium from acidic nitrate solutions with didodecylnaphthalenesulfonic acid synergized with bis(tert-butylbenzo)-21-crown-7. Anal Chem 64:3013–3017
Lamare V, Dozol JF, Fuangswasdi S, Neu FA, Thuéry P, Nierlich M, Asfari Z, Vicens J (1999) A new calix[4]arene-bis(crown ether) derivative displaying an improved caesium over sodium selectivity: molecular dynamics and experimental investigation of alkali-metal ion complexation. J Chem Soc Perkin Trans 2:271–284
Haverlock TJ, Bonnesen PV, Sachleben RA, Moyer BA (2000) Analysis of equilibria in the extraction of cesium nitrate by calix[4]arene-bis(t-octylbenzo-crown-6) in 1,2-dichloroethane. J Incl Phenom Macrocycl Chem 36:21–37
Dietz ML, Ensor DD, Harmon B, Seekamp S (2006) Separation and preconcentration of cesium from acidic media by extraction chromatography. J Sep Sci Technol 41:2083–2204
Ito T, Xu Y, Kim S-Y, Nagaishi R, Kimura T (2016) Adsorption behavior and radiation effects of a silica-based (Calix(4) + Dodecanol)/SiO2-P adsorbent for selective separation of Cs(I) from high level liquid waste. Sep Sci Technol 51:22–31
Raut DR, Mohapatra PK, Ansari SA, Manchanda VK (2008) Evaluation of a calix[4]-bis-crown-6 ionophore-based supported liquid membrane system for selective 137Cs transport from acidic solutions. J Membr Sci 310:229–236
Zhang A, Dai Y, Xu L, Chai Z (2013) Solvent extraction of cesium with a new compound calix[4]arene-bis[(4-methyl-1,2-phenylene)-crown-6]. J Chem Eng Data 58:3275–3281
Kumar V, Sharma JN, Achuthan PV, Hubli RC (2014) Selective separation of cesium from simulated high level liquid waste solution using 1,3-dioctyloxy calix[4]arene-benzo-crown-6. J Radioanal Nucl Chem 299:1547–1553
Gullion J, Sonnet P, Malval J-P, Massip S, Gosse I, Lager J-P, Lapouyade R, Rochette J, Monti J-P, Jarry C (2002) Synthesis and cesium binding affinity of new 25,27-Bis(alkyloxy)calix[4]arene-crown-6 conformers in relation to the alkyl pendent moiety. Supramol Chem 14:437
Visser AE, Swatloski RP, Reichert WM, Griffin ST, Rogers RD (2000) Traditional extractants in nontraditional solvents: groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind Eng Chem Res 39:3596–3604
Luo H, Dai S, Bonnesen PV, Buchanan AC, Holbrey JD, Bridges NJ, Rogers RD (2004) Extraction of cesium ions from aqueous solutions using calix[4]arene-bis(tert-octylbenzo-crown-6) in ionic liquids. Anal Chem 76:3078–3083
Luo H, Dai S, Bonnesen PV, Haverlock TJ, Moyer BA, Buchanan C (2006) A striking effect of ionic-liquid anions in the extraction of Sr2+ and Cs+ by dicyclohexano-18-Crown-6. Solv Extr Ion Exch 24:19–31
Ansari SA, Mohapatra PK, Raut DR, Manchanda VK (2011) Extraction of caesium(I) from HNO3 medium using room temperature ionic liquid containing calix[4]crown ligands as the selective extractants. Radiochim Acta 99:713–717
Takahashi T, Ito T, Kim S-Y (2016) Development of separation process of high-level liquid waste using extractant in ionic liquid extraction system. In: Proceedings of Asian Nuclear Prospects International Conference 2016
Kim S-Y, Xu Y, Ito T, Wu Y, Tada T, Hitomi K, Kuraoka E, Ishii K (2013) A novel partitioning process for treatment of high level liquid waste using macroporous silica-based adsorbents. J Radioanal Nucl Chem 295:1043–1050
Wu Y, Kim S-Y, Tozawa D, Hitomi K, Kuraoka E, Yamazaki H, Ishii K (2012) Study on selective separation of cesium from high level liquid waste using a macroporous silica-based supramolecular recognition absorbent. J Radioanal Nucl Chem 293:13–20
Takahashi T, Ito T, Kim S-Y, Tokuda H, Nagano N, Hitomi K (2016) Selective separation of Sr(II) from high level radioactive waste using crown ether in ionic liquid extraction system. J Ion Exch 27(2):21–26
Jagasia P, Mohapatra PK, Dhami PS, Gandhi PM, Wattal PK (2014) Evaluation of novel solvent systems containing calix-crown-6 ligands in a fluorinated solvent for cesium extraction from nitric acidic feeds. Sep Sci Technol 49:2151–2157
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 16H02444.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, T., Ito, T. & Kim, SY. Selective extraction of Cs(I) using 1,3-[(2,4-diethylheptylethoxy)oxy]-2,4-crown-6-calix[4]arene in ionic liquid solvents and its application to the treatment of high-level liquid waste. J Radioanal Nucl Chem 316, 1067–1073 (2018). https://doi.org/10.1007/s10967-018-5876-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5876-3