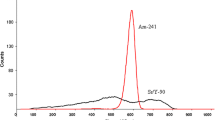
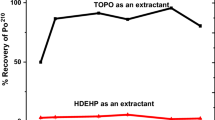
Abstract
The efficiency of ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA) in reducing the influence of salinity and extraction of iron and calcium into the organic phase along with uranium was studied. DTPA has been observed to be more suitable complexing agent compared to EDTA. Iron and calcium were found to be separated quantitatively with more than 95% recovery for uranium, facilitating its rapid and interference free analysis in the presence of DTPA. Uranium recovery under high salinity conditions was also observed to be in the range 89.7–98.6% in the presence of DTPA.



Similar content being viewed by others
References
Hess CT, Michel J, Horton TR, Prichard HM, Coniglio WA (1985) The occurrence of radioactivity in public water supplies in the United States. Health Phys 48:553–586
Sanchez-Cabeza JA, Pujol L (1995) A rapid method for the simultaneous determination of gross alpha and beta activities in water samples using a low background liquid scintillation counter. Health Phys 68:674–682
Tomé F, Sánchez A, Bejanaro J (1988) Activity ratios of natural uranium in surface waters. J Radioanal Nucl Chem Lett 126(6):419–427
Martínez-Aguirre A, García-León M (1994) Radionuclides in the environment in the south of Spain, anthropogenic enhancements due to industry. J Environ Radioact 22:155–159
APHA, AWWA and WEF (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, DC
Lally AE (1992) Chemical procedures. In: Ivanovich M, Harmon RS (eds) Uranium-series disequilibrium: applications to earth, marine, and environmental sciencies. Clarendon Press, Oxford, pp 95–126
Blackburn R, Al-Masri MS (1994) Determination of uranium by liquid scintillation and Cerenkov counting. Analyst 119:465–468
Krieger HL, Whittaker EL (1980) Uranium in drinking water-radiochemical method. method 908.0, prescribed procedures for measurement of radioactivity in drinking water. EPA-600/4-80-032
Mishra V, Das MK, Jeyakumar S, Sawant RM, Ramakumar KL (2011) Rapid separation and quantification of iron in uranium matrix by capillary zone electrophoresis (CZE). Am J Anal Chem 2:46–55
Abuzeida M, Arebi J, Zolatarev YA, Komarov NA (1987) Selective liquid scintillation method of uranium α-spectrometry. J Radioanal Nucl Chem 116:285
Jowzaee S (2013) Determination of selected natural radionuclide in Southwestern Caspian groundwater using liquid scintillation counting. Radiat Prot Dosim 157(2):234–241
Sanjukta KA, Niyoti S, Shenoy SP, Sounderajan S, Venkateswaran G (2008) Direct determination of uranium in seawater by laser fluorimetry. Talanta 77:422–426
Abbasisiar F, Hosseini T, Fathivand A, Heravi G (2004) Determination of uranium isotopes (234U, 238U) and natural uranium (U-nat) in water samples by alpha spectrometry. J Radiat Res 2(1):1–6
Endrizzi F, Leggett CJ, Rao L (2016) Scientific basis for efficient extraction of uranium from seawater. I: understanding the chemical speciation of uranium under seawater conditions. Eng Chem Res 55(15):4249–4256. doi:10.1021/acs.iecr.5b03679
Aupiais J (2004) Rapid determination of uranium activity and concentration in water by alpha liquid scintillation with α/β discrimination. J Anal Chim Acta 517:221–228
Reddy JP, Pulhani V, Dhole SD, Bhade SPD, Anilkumar S, Kolekar RV, Singh R (2016) Application of extractive liquid scintillation spectrometryfor rapid determination of uranium. J Radioanal Nucl Chem. doi:10.1007/s10967-016-4698-4
Hennig C, Tutschku J, Rossberg A, Bernhard G, Scheinost AC (2005) Comparative EXAFS Investigation of uranium(VI) and –(IV) aquo chloro complexes in solution using a newly developed spectroelectrochemical cell. Inorg Chem 44:6655–6661. doi:10.1021/ic048422n
Liu W, Etschmann B, Brugger J, Spiccia L, Foran G, McInnes B (2006) UV-Vis spectrophotometric and XAFS studies of ferric chloride complexes in hyper-saline LiCl solutions at 25-90 °C. Chem Geol 231:326–349. doi:10.1016/j.chemgeo.2006.02.005
Duffey JM (1997) Development of rapid procedure for the measurement of uranium in drinking water by PEARLS spectrometry. J Radioanal Nucl Chem 221:115–122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, P.J., Pulhani, V., Dhole, S.D. et al. Studies on matrix interferences during uranium analysis by extractive liquid scintillation technique. J Radioanal Nucl Chem 311, 1923–1927 (2017). https://doi.org/10.1007/s10967-016-5140-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5140-7