Abstract
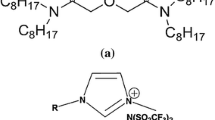
Extraction of europium(III) from nitric acid medium was studied in a solution of N,N,N′,N′-tetra-n-octyldiglycolamide (TODGA) dissolved in 1-methyl-3-octylimidazolium bis(trifluormethanesulfonyl)imide ([C8mim][NTf2]) ionic liquid at higher Eu(III) loading conditions ranging from 1 to 100 g/L Eu(III) in aqueous phase. The extraction of Eu(III) was studied as a function of various parameters such as the concentration of nitric acid, Eu(III) ion in aqueous phase, TODGA, nature of the ionic liquid, nature of extractant, and phase modifiers etc. The results revealed that Eu(III) could be loaded to the extent of 1: 3 stoichiometry in ionic liquid phase without leading to undesirable third phase formation.









Similar content being viewed by others
References
Vasudeva Rao PR, Kolarik Z (1996) A review of third phase formation in extraction of actinides by neutral organophosphorus extractants. Solv Extr Ion Exch 14:955–993
Suresh A, Srinivasan TG, Vasudeva Rao PR (2009) Parameters Influencing third phase formation in the extraction of Th(NO3)4 by some trialkyl phosphates. Solv Extr Ion Exch 27:132–158
Gujar RB, Ansari SR, Mohapatra PK, Manchanda VK (2010) Development of T2EHDGA based process for actinide partitioning. Part I: batch studies for process optimization. Solv Extr Ion Exch 28:350–366
Tachimori S, Sasaki Y, Suzuki S (2002) Modification of TODGA in n -dodecane solvent with a monoamide for high loading of lanthanides(III) and actinides(III). Solv Extr Ion Exch 20:687–699
Deepika P, Sabharwal KN, Srinivasan TG, Vasudeva Rao PR (2010) Studies on the use of N, N, N, N-tetra (2-ethylhexyl) diglycolamide (TEHDGA) for actinide partitioning I: investigation on third-phase formation and extraction behavior. Solv Extr Ion Exch 28:184–201
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99:2071–2084
Sengupta A, Murali MS, Mohapatra PK (2013) Role of alkyl substituent in room temperature ionic liquid on the electrochemical behavior of uranium ion and its local environment. J Radioanal Nucl Chem 298:209–217
Rama R, Kumaresan R, Venkatesan KA, Antony MP, Vasudeva Rao PR (2014) Insights into the extraction behavior of U(VI) in Aliquat-336 based ionic liquids. Radiochim Acta 102:1009–1016
Kumaresan R, Jain R, Venkatesan KA, Antony MP, Bhanage BM (2015) Extraction and electrochemical behavior of fission palladium in room-temperature ionic liquid. J Radioanal Nucl Chem 303:1047–1052
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2011) Room temperature ionic liquid diluent for the extraction of Eu(III) using TRUEX extractants. J Radioanal Nucl Chem 290:215–219
Dietz ML, Stepinski DC (2008) Anion concentration-dependent partitioning mechanism in the extraction of uranium into room-temperature ionic liquids. Talanta 75:598–603
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2010) Unusual extraction of plutonium(IV) from uranium(VI) and americium(III) using phophonate based task specific ionic liquid. Radiochim Acta 98:459–466
Giridhar P, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2008) Extraction of uranium(VI) by 1.1 M tri-n-butyl phosphate/ionic liquid and the feasibility of recovery by direct electro deposition from organic phase. J Alloys Compd 448:104–108
Nikitenko SI, Cannes C, Naour CL, Moisy P, Trubert D (2005) Spectroscopic and electrochemical studies of U(IV)-hexachloro complexes in hydrophobic room-temperature ionic liquids [BuMeIm][Tf2N] and[MeBu3N][Tf2N]. Inorg Chem 44:9497–9505
Giridhar P, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2007) Electrochemical behavior of uranium(VI) in 1-butyl-3-methylimidazoliumchloride and thermal characterization of uranium oxide deposit. Electrochim Acta 52:3006–3012
Vasudeva Rao PR, Venkatesan KA, Rout A, Srinivasan TG, Nagarajan K (2012) Potential applications of room temperature ionic liquids for fission products and actinide separation. Sep Sci Technol 47:204–222
Binnemans K (2007) Lanthanides and actinides in ionic liquids. Chem Rev 107:2592–2614
Billard I, Ouadi A, Gaillard C (2011) Liquid–liquid extraction of actinides, lanthanides and fission products by use of ionic liquids: from discovery to understanding. Anal Bioanal Chem 400:1555–1566
Sun X, Luo H, Dai S (2011) Ionic liquids based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem Rev 112:2100–2128
Visser AE, Swatloski RP, Reichert WM, Griffin ST, Rogers D (2000) Traditional extractants in non-traditional solvents: group1 and 2 extraction by crown ethers in room temperature ionic liquids. Ind Eng Chem Res 39:3596–3604
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2011) Extraction and third phase formation behavior of Eu(III) in CMPO–TBP extractants present in room temperature ionic liquid. Sep Purif Technol 76:238–243
Ansari SA, Pathak PN, Mohapatra PK, Manchanda VK (2011) Chemistry of diglycolamides: promising extractants for actinide partitioning. Chem Rev 112:1751–1772
Magnusson D, Christiansen B, Glatz JP, Malmeck R, Modolo G, Purroy DS, Sorel C (2009) Demonstration of a TODGA based extraction process for the partitioning of minor actinides from a P;UREX raffinate. Solv Extr Ion Exch 27:26–35
Mowafy EA, Mohamed D (2014) Extraction behavior of trivalent lanthanides from nitric acid medium by selected structurally related diglycolamides as novel extractants. Sep Purif Technol 128:18–24
Modolo G, Asp H, Schreinemachers C, Vijgen H (2007) Development of TODGA based process for partitioning of actinides from purex raffinate part-I: batch extraction optimization studies and stability test. Solv Extr Ion Exch 25:703–721
Ravi J, Suneesh AS, Prathibha Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2011) Extraction behavior of some actinides and fission products from nitric acid medium by a new unsymmetrical diglycolamide. Solv Extr Ion Exch 29:86–105
Ravi J, Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2014) Feasibility of using Di-dodecyl-Di-octyl diglycolamide for partitioning of minor actinides from fast reactor high-level liquid waste. Solv Extr Ion Exch 32:424–436
Nayak PK, Kumaresan R, Venkatesan KA, Rajeswari S, Subramanian CGS, Antony MP, Vasudeva Rao PR (2013) Single-cycle separation of americium(III) from simulated high-level liquid waste using tetra-bis(2-ethylhexyl)diglycolamide and bis(2-ethylhexyl)phosphoric acid solution. J Environ Chem Eng 1:559–565
Shimojo K, Kurahashi K, Naganawa H (2008) Extraction behavior of lanthanides using a diglycolamide derivative TODGA in ionic liquids. Dalton Trans 37:5083–5088
Prathibha T, Venkatesan KA, Robert Selvan B, Antony MP, Vasudeva Rao PR (2012) Anomalous extraction behavior of americium(III) in some diglycolamide isomers present in ionic liquid medium. Radiochim Acta 100:907–913
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rama, R., Rout, A., Venkatesan, K.A. et al. Loading behavior of Eu(III) at high aqueous concentrations in diglycolamide/ionic liquid systems. J Radioanal Nucl Chem 308, 835–842 (2016). https://doi.org/10.1007/s10967-015-4568-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4568-5