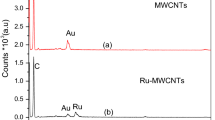
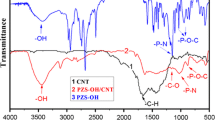
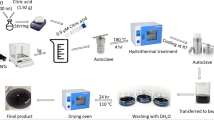
Abstract
The adsorption of UO2 2+ ions from aqueous solution using ethylene diamine and triazine functionalized multiwall carbon nanotubes have been investigated. The result for kinetic study within 90 min revealed that with increasing the time, uranium adsorption increased and process followed pseudo first order model. Isotherm study was investigated using Langmuir and Freundlich model as maximum adsorption capacity by ethylene diamine and triazine was 69.44 and 74.62 mg g−1 respectively. Thermodynamic parameters shows negative value of Gibbs free energy and endothermic pathway for uranium adsorption. These results indicate that prepared sorbent has good efficiency for uranium adsorption from aqueous solutions.






Similar content being viewed by others
References
Xu J, Chen M, Zhang C, Yi Z (2013) Adsorption of uranium(VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan. J Radioanal Nucl Chem 298:1375–1383
Cao Q, Liu Y, Kong X, Zhou L, Guo H (2013) Synthesis of phosphorus-modified poly(styrene-co-divinylbenzene) chelating resin and its adsorption properties of uranium(VI). J Radioanal Nucl Chem 298(1137):1147
Aly Z, Luca V (2013) Uranium extraction from aqueous solution using dried and pyrolyzed tea and coffee wastes. J Radioanal Nucl Chem 295:889–900
Belgacem A, Rebiai R, Hadoun H, Khemaissia S, Belmedani M (2013) The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ Sci Pollut Res 21:684–694
Villalobos-Rodriguez R, Montero-Cabrera ME, Esparza-Ponce HE, Herrera-Peraza EF, Ballinas-Casarrubias ML (2012) Uranium removal from water using cellulose triacetate membranes added with activated carbon. Appl Rad Isot 70:872–881
Bonato M, Ragnarsdottir KV, Allen GC (2012) Removal of uranium(VI), lead(II) at the surface of TiO2 nanotubes studied by X-ray photoelectron spectroscopy. Water Air Soil Pollut 223:3845–3857
Nie B, Zhang Z, Cao X, Liu Y, Liang P (2013) Sorption study of uranium from aqueous solution on ordered mesoporous carbon CMK-3. J Radioanal Nucl Chem 295:663–670
Song M, Wang Q, Meng Y (2012) Removal of UO2 2+ from aqueous solution by plasma functionalized MWCNTs. J Radioanal Nucl Chem 293:899–906
Rahmati A, Ghaemi A, Samadfam M (2012) Kinetic and thermodynamic studies of uranium(VI) adsorption using Amberlite IRA-910 resin. Ann Nucl Energy 39:42–48
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci 296:434–441
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P, Zhang M (2012) Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation. Chem Eng J 198–199:412–419
Zhang X, Lanyang JL, Wanga J, Li R, Liu Q, Zhang M, Liu L (2012) Removal of uranium(VI) from aqueous solutions by magnetic Mg–Al layered double hydroxide intercalated with citrate: kinetic and thermodynamic investigation. Colloids Surf A 414:220–227
Anirudhan TS, Bringle CD, Rijith S (2010) Removal of uranium(VI) from aqueous solutions and nuclear industry effluents using humic acid-immobilized zirconium-pillared clay. J Environ Radioact 101:267–276
Alpaslan D, Aktas N, Yilmaz S, Sahiner N, Guven O (2014) The preparation of p(acrylonitrile-co-acrylamide) hydrogels for uranyl ion recovery from aqueous environments. Hacet J Biol Chem 42:89–97
Ozeroglu C, Metin N (2012) Adsorption of uranium ions by crosslinked polyester resin functionalized with acrylic acid from aqueous solutions. J Radioanal Nucl Chem 292:923–935
Donat R (2009) The removal of uranium (VI) from aqueous solutions onto natural sepiolite. J Chem Thermodyn 41:829–835
Shuibo X, Chun Z, Xinghuo Z, Jing Y, Xiaojian Z, Jingsong W (2009) Removal of uranium (VI) from aqueous solution by adsorption of hematite. J Environ Radioact 100:162–166
Weihua Z, Lei Z, Runping H (2009) Removal of uranium (VI) by fixed bed ion-exchange column using natural zeolite coated with manganese oxide. Chin J Chem Eng 17:585–593
Zhang ZB, Yu XF, Cao XH, Hua R, Li M, Liu YH (2014) Adsorption of U(VI) from aqueous solution by sulfonated ordered mesoporous carbon. J Radioanal Nucl Chem 301:821–830
Selcuk S, Ersen Y, Ali B (2013) Amine-modified maleic anhydride containing terpolymers for the adsorption of uranyl ion in aqueous solutions. J Radioanal Nucl Chem 298:923–930
Yousif AM, El-Afandy AH, Abdel Wahab GM, Mubark AE, Ibrahim IA (2015) Selective separation of uranium(VI) from aqueous solutions using amine functionalized cellulose. J Radioanal Nucl Chem 303:1821–1833
Wang M, Qiu J, Tao X, Wu C, Cui W, Liu Q, Lu S (2011) Effect of pH and ionic strength on U(IV) sorption to oxidized multiwalled carbon nanotubes. J Radioanal Nucl Chem 288:895–901
Chisholm-Brause CJ, Berg JM, Matzner RA, Morris DE (2001) Uranium(VI) sorption complexes on montmorillonite as a function of solution chemistry. J Colloid Interface Sci 233:38–49
Peng GW, Ding DX, Xiao FZ, Wang XL, Hun N, Wang YD, Dai YM, Cao Z (2014) Adsorption of uranium ions from aqueous solution by amine-group functionalized magnetic Fe3O4 nanoparticle. J Radioanal Nucl Chem 301:781–788
Kuo CY (2009) Comparison with as-grown and microwave modified carbon nanotubes to removal aqueous bisphenol A. Desalination 249:976–982
Liu Y, Gao L, Sun J, Wang Y (2010) Functionalization of carbon nanotubes for nanoparticle attachment. J Ceram Process Res 11:120–122
Ho YS, Mckay G (2002) Application of kinetic models to the sorption of copper(II) on to Peat. Ads Sci Technol 20:797–815
Lin SH, Juang RS (2002) Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J Hazard Mater 92:315–326
Chowdhury S, Saha P (2011) Adsorption thermodynamics and kinetics of malachite green onto Ca(OH)2 treated fly ash. J Environ Eng 137:388–397
Ozdemir G, Yapar S (2009) Adsorption and desorption behavior of copper ions on Na-montmorillonite: effect of rhamnolipids and pH. J Hazard Mater 166:1307–1313
Naiya TK, Bhattacharya AK, Das SK (2009) Clarified sludge (basic oxygen furnace sludge): an adsorbent for removal of Pb(II) from aqueous solutions: kinetics, thermodynamics and desorption studies. J Hazard Mater 170:252–262
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alijani, H., Beyki, M.H. & Mirzababaei, S.N. Adsorption of UO2 2+ ions from aqueous solution using amine functionalized MWCNT: kinetic, thermodynamic and isotherm study. J Radioanal Nucl Chem 306, 165–173 (2015). https://doi.org/10.1007/s10967-015-4078-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4078-5