Abstract
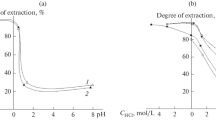
Sorption of niobium on silica colloids was studied using radiotracer technique in the pH range of 2–11 in NaClO4 medium. Silica was characterized using X-ray diffraction, light scattering and surface area measurements. The point of zero charge for silica colloids was about pH 2. The sorption of niobium was >99 % in the pH range of 3–9 and was slightly lower (~95 %) below pH 3 and above pH 9. The quantitative sorption in the pH range 3–9 could be explained by surface complexation model. The small reduction in sorption was attributed to the existence of small fraction of niobium as cationic species at pH ≤ 2 and anionic species above pH 9. Presence of humic acid was found to have negligible effects on the sorption characteristics.






Similar content being viewed by others
References
Howe-Grant M (1996) Kirk–Othmer encyclopedia of chemical technology, vol 17, 4th edn. Wiley, New York
Lister DH (1992) Coolant technology for water cooled reactors. IAEA-TECHDOC-667, vol. 2, IAEA, Vienna
Remya Devi PS, Joshi S, Verma R, Reddy AVR, Lali AM, Gantayet LM (2010) Ion-exchange separation of 60Co and 125Sb from zirconium for radioactive waste management. Nucl Technol 171:220–227
Gangotra S, Ouseph PM, Anantharaman S, Sahoo KC (2002) Gamma spectrometry studies on irradiated zircaloy pressure tubes. In: ZIRC-2002, BARC, Mumbai, India, p 658
Geological disposal of radioactive waste (1984) An overview of the current status of understanding and development. Organization for economic cooperation and development (OECD), Paris
Basu H, Singhal RK, Pimple MV, Kumar A, Reddy AVR (2014) Association and migration of uranium and thorium with silica colloidal particles in saturated subsurface zone. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3677-x
Honeyman BD (1999) Colloidal culprits in contamination. Nature 397:23–24
Kersting AB, Efurd DW, Finnegan DL, Rokop DJ, Smith DK, Thompson JL (1999) Migration of plutonium at Nevada test site. Nature 397:56–59
McCarthy JF, Zachara JM (1989) Subsurface transport of contaminants. Environ Sci Technol 23:496–502
Buffle J, Leppard GG (1995) Characterization of aquatic colloids and macromolecules. 1. Structure and behavior of colloidal material. Environ Sci Technol 29:2169–2175
Sachs S, Bernhard G (2011) Influence of humic acids on the actinide migration in the environment: suitable humic acid model substances and their application in studies with uranium-a review. J Radioanal Nucl Chem 290:17–29
Buckau G (2005) Humic substances in performance assessment of nuclear waste disposal: actinide and iodine migration in the far field. 3rd Technical progress report. FZKA 7070
Geckeis H, Stumpf T (2009) Institute for nuclear waste disposal. Annual Report. KIT-SR 7559
Lieser KH (1995) Radionuclides in geosphere: sources, reactions in natural waters and interaction with solids. Radiochim Acta 70(71):355–376
Silva RJ, Nitsche H (1995) Actinide environmental chemistry. Radiochim Acta 70(71):377–396
Kumar S, Tomar BS, Ramanathan S, Manchanda VK (2006) Effect of humic acid on cesium sorption on silica colloids. Radiochim Acta 94:369–373
Pathak PN, Choppin GR (2007) Kinetics and thermodynamics of uranium (VI) sorption on hydrous silica. Radiochim Acta 95:507–512
Skoog DA, West DM, Holler FJ, Crouch SR (2004) Fundamentals of analytical chemistry, 4th edn. Brooks/Cole, Boston, p 389
Stevenson FJ (1982) Humus chemistry: genesis, composition, reactions. Wiley, New York
Kraus KA, Moore GE (1951) Anion exchange studies I. Separation of zirconium and niobium in HCl–HF mixtures. J Am Chem Soc 73:9–13
Stumm W, Morgan JJ (1970) An introduction emphasizing chemical equilibria in natural waters. Wiley Interscience, New York 481
Samadfam M, Jintoku T, Ohashi H, Mitsugashira T, Hara M, Suzuki Y (2000) Effects of humic acid on the sorption of Am(III) and Cm(III) on kaolinite. Radiochim Acta 88:717–721
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley, New York p 897
Gibalo IM (1968) Analytical chemistry of niobium and tantalum. Israel program for scientific translations, Jerusalem, p 9
Caletka R (1970) Separation of 95Zr and 95Nb by means of sorption on silica gel from hydrochloric acid solutions. J Radioanal Nucl Chem 6:5–10
Stumm W, Morgan JJ (1996) Aquatic chemist. Wiley, New York, p 516
Burcham JB, Datka J, Wachs IE (1999) In situ vibrational spectroscopy studies of supported niobium oxide catalysts. J Phys Chem B 103:6015–6024
Acknowledgments
Authors thank Dr. P. A. Hassan for particle size measurement, Dr. A.K. Tyagi for X-ray diffraction measurement and Dr. S. Ramanathan for zeta potential measurement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, M., Remya Devi, P.S., Verma, R. et al. Sorption of niobium on colloidal silica and the effect of humic acid. J Radioanal Nucl Chem 306, 147–153 (2015). https://doi.org/10.1007/s10967-015-4055-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4055-z